Noretynodrel (BAN, Rinn) 3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pp375-430-Annex 1.Qxd
ANNEX 1 CHEMICAL AND PHYSICAL DATA ON COMPOUNDS USED IN COMBINED ESTROGEN–PROGESTOGEN CONTRACEPTIVES AND HORMONAL MENOPAUSAL THERAPY Annex 1 describes the chemical and physical data, technical products, trends in produc- tion by region and uses of estrogens and progestogens in combined estrogen–progestogen contraceptives and hormonal menopausal therapy. Estrogens and progestogens are listed separately in alphabetical order. Trade names for these compounds alone and in combination are given in Annexes 2–4. Sales are listed according to the regions designated by WHO. These are: Africa: Algeria, Angola, Benin, Botswana, Burkina Faso, Burundi, Cameroon, Cape Verde, Central African Republic, Chad, Comoros, Congo, Côte d'Ivoire, Democratic Republic of the Congo, Equatorial Guinea, Eritrea, Ethiopia, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Kenya, Lesotho, Liberia, Madagascar, Malawi, Mali, Mauritania, Mauritius, Mozambique, Namibia, Niger, Nigeria, Rwanda, Sao Tome and Principe, Senegal, Seychelles, Sierra Leone, South Africa, Swaziland, Togo, Uganda, United Republic of Tanzania, Zambia and Zimbabwe America (North): Canada, Central America (Antigua and Barbuda, Bahamas, Barbados, Belize, Costa Rica, Cuba, Dominica, El Salvador, Grenada, Guatemala, Haiti, Honduras, Jamaica, Mexico, Nicaragua, Panama, Puerto Rico, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Suriname, Trinidad and Tobago), United States of America America (South): Argentina, Bolivia, Brazil, Chile, Colombia, Dominican Republic, Ecuador, Guyana, Paraguay, -

61/2003, Uredbeni
Stran 7380 / Št. 61 / 26. 06. 2003 Uradni list Republike Slovenije ODDELEK VI PROIZVODI KEMIČNE INDUSTRIJE ALI PODOBNIH INDUSTRIJ OPOMBE: 1. (a) Proizvodi (razen radioaktivnih rud), ki ustrezajo poimenovanjem v tar. št. 2844 ali 2845, se uvrščajo v omenjeni tarifni številki, ne pa v druge tariFne številke Nomenklature. (b) V skladu s prejšnjim odstavkom se proizvodi, ki ustrezajo poimenovanjem v tar.št. 2843 ali 2846., uvrščajo samo v omenjeni tarifni številki, ne pa v druge tariFne številke tega oddelka. 2. V skladu z opombo št.1 se proizvodi, ki bi se zaradi pakiranja na drobno ali v odmerjene količine uvrstili v tar. št. 3004, 3005, 3006, 3212, 3303, 3304, 3305, 3306, 3307, 3506, 3707 ali 3808, se uvrščajo samo v omenjeni tar. št., (2844 ali 2845) ne pa v druge tariFne številke Nomenklature. 3. Proizvodi, pripravljeni v garniturah, ki sestoje iz dveh ali več ločenih sestavin, od katerih se neka- tere ali vse uvrščajo v ta oddelek in so namenjene za to, da se pomešajo skupaj, da bi se dobil proizvod VI. ali VII. oddelka, se uvrščajo v ustrezno tarifno številko za ta proizvod pod pogojem, da so sestavine: (a) take, da je iz tega, kako so pripravljene, razvidno, da so namenjene za uporabo skupaj brez poprejšnjega prepakiranja, (b) da se skupaj carinijo in (c) da so take, da je razvidno iz njihove narave ali po sorazmernih količinah, v katerih so zasto- pane, da se med seboj dopolnjujejo. SPLOŠNA DOLOČILA Opomba št. 1 Skladno z določili točke (a) opombe št. 1 se vsi radioaktivni kemični elementi, radioaktivni izotopi in spojine teh elementov in izotopov (anorganskih ali organskih, kemično določenih ali nedoločenih) uvrščajo v tar. -

MMC International BV
M.M.C. International Steroid Substances Steroid Test A Colour Steroid Test B Colour Steroid Test B Colour with UV Light Stanozolol/ Oxandrolone Test Clenbuterol/ Oxymetholone Test Ephedrine Test Alfadolone Orange Yellow Nil - - - Androsterone Orange Yellow White - - - Beclometasone Brown–yellow Orange Nil - - - Betamethasone Orange–brown Pink–Orange Nil - - - Boldenone Base (Equipoise, Ganabol) (pure powder) Warm red after 2 min. Dark Orange after 2 min. Bright Light Orange - - - Boldenone Undecanoate (oil) Dark brownish-red Dark Red Bright Light Orange - - - Boldenone Undecylenate (oil) Orange - Light Brown Dark Orange → Brown Bright Light Orange-Yellow - - - Carbenoxolone (CBX) Orange Yellow Yellow - - - Cholesterol Violet Orange White - - - Clenbuterol (Spiropent, Ventipulmin) - - - - Purple - Dark brown with yellow-green on the Dark brown with yellow-green on the Clomiphene (Androxal, Clomid, Omifin) Nil Dark brown to black No reaction Dark brown to black sides of the ampoule sides of the ampoule Cortisone Orange Yellow Green - - - Desoxycortone Blue–black Yellow Yellow - - - Dexamethasone Yellow Orange–pink Nil - - - Dienestrol Yellow Orange–red Nil - - - Diethylstilbestrol (DES) Orange (→yellow–green) Nil - - - Dimethisterone Brown–green Orange–red Yellow - - - Drostanolone Propionate (Masteron) (oil) Bright green Yellow-Orange Orange - - - Dydrogesterone (Duphaston) - Orange Green-Yellow - - - Enoxolone Orange Yellow Green-Yellow - - - Ephedrine (also for Pseudo- and Nor-Ephedrine) - - - - - Orange Estradiol (Oestradiol) Orange -

ESTROSTEP Fe (Norethindrone Acetate and Ethinyl Estradiol Tablets, USP and Ferrous Fumarate Tablets*) *Ferrous Fumarate Tablets Are Not USP for Dissolution and Assay
ESTROSTEP Fe (Norethindrone Acetate and Ethinyl Estradiol Tablets, USP and Ferrous Fumarate Tablets*) *Ferrous fumarate tablets are not USP for dissolution and assay. ESTROSTEP® Fe (Each white triangular tablet contains 1 mg norethindrone acetate and 20 mcg ethinyl estradiol; each white square tablet contains 1 mg norethindrone acetate and 30 mcg ethinyl estradiol; each white round tablet contains 1 mg norethindrone acetate and 35 mcg ethinyl estradiol; each brown tablet contains 75 mg ferrous fumarate.) Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases. DESCRIPTION ESTROSTEP® Fe is a graduated estrophasic oral contraceptive providing estrogen in a graduated sequence over a 21-day period with a constant dose of progestogen. ESTROSTEP Fe provides for a continuous dosage regimen consisting of 21 oral contraceptive tablets and seven ferrous fumarate tablets. The ferrous fumarate tablets are present to facilitate ease of drug administration via a 28-day regimen, are non-hormonal, and do not serve any therapeutic purpose. Each white triangle-shaped tablet contains 1 mg norethindrone acetate [(17 alpha)-17- (acetyloxy)-19-norpregna-4-en-20-yn-3-one] and 20 mcg ethinyl estradiol [(17 alpha)-19- norpregna-1,3,5(10)-trien-20-yne-3,17-diol]; each white square-shaped tablet contains 1 mg norethindrone acetate and 30 mcg ethinyl estradiol; and each white round tablet contains 1 mg norethindrone acetate and 35 mcg ethinyl estradiol. Each tablet also contains calcium stearate; lactose; microcrystalline cellulose; and starch. The structural formulas are as follows: Each brown tablet contains ferrous fumarate, mannitol, povidone, microcrystalline cellulose, sodium starch glycolate, magnesium stearate, sucralose and spearmint flavor. -

Combined Estrogen–Progestogen Menopausal Therapy
COMBINED ESTROGEN–PROGESTOGEN MENOPAUSAL THERAPY Combined estrogen–progestogen menopausal therapy was considered by previous IARC Working Groups in 1998 and 2005 (IARC, 1999, 2007). Since that time, new data have become available, these have been incorporated into the Monograph, and taken into consideration in the present evaluation. 1. Exposure Data 1.1.2 Progestogens (a) Chlormadinone acetate Combined estrogen–progestogen meno- Chem. Abstr. Serv. Reg. No.: 302-22-7 pausal therapy involves the co-administration Chem. Abstr. Name: 17-(Acetyloxy)-6-chlo- of an estrogen and a progestogen to peri- or ropregna-4,6-diene-3,20-dione menopausal women. The use of estrogens with IUPAC Systematic Name: 6-Chloro-17-hy- progestogens has been recommended to prevent droxypregna-4,6-diene-3,20-dione, acetate the estrogen-associated risk of endometrial Synonyms: 17α-Acetoxy-6-chloro-4,6- cancer. Evidence from the Women’s Health pregnadiene-3,20-dione; 6-chloro-Δ6-17- Initiative (WHI) of adverse effects from the use acetoxyprogesterone; 6-chloro-Δ6-[17α] of a continuous combined estrogen–progestogen acetoxyprogesterone has affected prescribing. Patterns of exposure Structural and molecular formulae, and relative are also changing rapidly as the use of hormonal molecular mass therapy declines, the indications are restricted, O CH and the duration of the therapy is reduced (IARC, 3 C 2007). CH3 CH3 O C 1.1 Identification of the agents CH3 H O 1.1.1 Estrogens HH For Estrogens, see the Monograph on O Estrogen-only Menopausal Therapy in this Cl volume. C23H29ClO4 Relative molecular mass: 404.9 249 IARC MONOGRAPHS – 100A (b) Cyproterone acetate Structural and molecular formulae, and relative Chem. -

Emergency Contraception
Need more information? State of Illinois This document provides some very basic information about emergency Illinois Department of Public Health contraception and how it works. If EC is something you want to know more about, ask the hospital emergency personnel about their policy on EC or ask the sexual assault advocate for assistance in getting this information. Local Support/Assistance: What You Should Know About Emergency Contraception For more information, contact ILLINOIS DEPARTMENT OF PUBLIC HEALTH 535 W. Jefferson St. Springfield, IL 62761 217-782-5750 Women’s Health-line 888-522-1282 TTY (hearing impaired use only) 800-547-0466 www.idph.state.il.us Developed by the Illinois Department of Public Health in cooperation with the Illinois Coalition Against Sexual Assault Printed by Authority of the State of Illinois IOCI 0041-1 Illinois law provides that victims of sexual assault are entitled to If I am already pregnant, medically and factually accurate information about emergency contra - ception (EC) when they receive emergency care in a hospital. Under will EC hurt the fetus? the Sexual Assault Survivors Emergency Treatment Act (410 ILCS There is no evidence that EC causes birth defects. However, there 70/2.2), Illinois hospitals are required to have a policy in place regarding have been no studies specific to taking birth control at this dosage. emergency contraception. Individual hospital protocols must ensure What is known is that babies born to women who continue taking that each victim of sexual assault will receive medically and factually birth control pills before finding out they are pregnant do not have accurate written and oral information about emergency contraception; higher rates of birth defects. -

ARANELLE- Norethindrone and Ethinyl Estradiol Teva Pharmaceuticals USA, Inc
ARANELLE- norethindrone and ethinyl estradiol Teva Pharmaceuticals USA, Inc. ---------- Aranelle®(norethindrone and ethinyl estradiol tablets USP) Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases. DESCRIPTION Aranelle® 28-Day Regimen (norethindrone and ethinyl estradiol tablets USP) provides a continuous oral contraceptive regimen of 7 light yellow tablets, 9 white tablets, 5 more light yellow tablets, and then 7 peach tablets. Each light yellow tablet contains norethindrone, USP 0.5 mg and ethinyl estradiol, USP 0.035 mg, each white tablet contains norethindrone, USP 1 mg and ethinyl estradiol, USP 0.035 mg, and each peach tablet contains inert ingredients. Norethindrone, USP is a potent progestational agent with the chemical name 17- Hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one. Ethinyl estradiol, USP is an estrogen with the chemical name 19-Nor-17α-pregna-1,3,5(10)-trien-20-yne-3,17-diol. Their structural formulae follow. Norethindrone, USP Ethinyl Estradiol, USP The light yellow tablet contains the following inactive ingredients, D&C yellow no. 10 aluminum lake, lactose monohydrate, magnesium stearate, and pregelatinized starch. The white tablet contains the following inactive ingredients, lactose monohydrate, magnesium stearate, and pregelatinized starch. The inactive peach tablets contain the following inactive ingredients, anhydrous lactose, FD&C yellow no. 6 aluminum lake, magnesium stearate, microcrystalline cellulose, and pregelatinized starch. CLINICAL PHARMACOLOGY Combination oral contraceptives act by suppression of gonadotrophins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which may reduce the likelihood of implantation). -

Emergency Contraception
AQ FREQUENTLY ASKED QUESTIONS FAQ114 fCONTRACEPTION Emergency Contraception • What is emergency contraception? • How does emergency contraception work? • What are the different types of emergency contraception? • What is the most effective form of emergency contraception? • How does the copper intrauterine device work? • What are the possible side effects of using the copper intrauterine device? • How do emergency contraception pills work? • How often can I use emergency contraception pills? • What are the possible side effects of taking emergency contraception pills? • Is there anything that decreases the effectiveness of emergency contraception pills? • How can I get emergency contraception as soon as possible? • How do I start or resume using a birth control method after taking emergency contraception pills? • Do I need follow-up care after using emergency contraception? • Glossary What is emergency contraception? Emergency contraception (EC) reduces the chance of pregnancy after unprotected sexual intercourse. Common situations in which EC could be used include forgetting to take several birth control pills in a row, having a condom break or slip off, or not using a birth control method during sex. It also can be used after a woman has been raped. How does emergency contraception work? Using EC does not cause an abortion. An abortion ends an existing pregnancy. EC prevents pregnancy from occurring. EC must be used soon after unprotected sexual intercourse to be effective. It does not work if pregnancy has already occurred. What are the different types of emergency contraception? There are two main types of EC: 1) the copper intrauterine device (IUD) and 2) EC pills. There are three types of EC pills: 1) ulipristal, 2) progestin-only pills, and 3) combined EC pills. -

(ESI) for Integrative Biology. This Journal Is © the Royal Society of Chemistry 2017
Electronic Supplementary Material (ESI) for Integrative Biology. This journal is © The Royal Society of Chemistry 2017 Table 1 Enriched GO terms with p-value ≤ 0.05 corresponding to the over-expressed genes upon perturbation with the lung-toxic compounds. Terms with corrected p-value less than 0.001 are shown in bold. GO:0043067 regulation of programmed GO:0010941 regulation of cell death cell death GO:0042981 regulation of apoptosis GO:0010033 response to organic sub- stance GO:0043068 positive regulation of pro- GO:0010942 positive regulation of cell grammed cell death death GO:0006357 regulation of transcription GO:0043065 positive regulation of apop- from RNA polymerase II promoter tosis GO:0010035 response to inorganic sub- GO:0043066 negative regulation of stance apoptosis GO:0043069 negative regulation of pro- GO:0060548 negative regulation of cell death grammed cell death GO:0016044 membrane organization GO:0042592 homeostatic process GO:0010629 negative regulation of gene ex- GO:0001568 blood vessel development pression GO:0051172 negative regulation of nitrogen GO:0006468 protein amino acid phosphoryla- compound metabolic process tion GO:0070482 response to oxygen levels GO:0045892 negative regulation of transcrip- tion, DNA-dependent GO:0001944 vasculature development GO:0046907 intracellular transport GO:0008202 steroid metabolic process GO:0045934 negative regulation of nucle- obase, nucleoside, nucleotide and nucleic acid metabolic process GO:0006917 induction of apoptosis GO:0016481 negative regulation of transcrip- tion GO:0016125 sterol metabolic process GO:0012502 induction of programmed cell death GO:0001666 response to hypoxia GO:0051253 negative regulation of RNA metabolic process GO:0008203 cholesterol metabolic process GO:0010551 regulation of specific transcrip- tion from RNA polymerase II promoter 1 Table 2 Enriched GO terms with p-value ≤ 0.05 corresponding to the under-expressed genes upon perturbation with the lung-toxic compounds. -

Emergency Contraception Krishna K
POLICY STATEMENT Organizational Principles to Guide and Define the Child Health Care System and/or Improve the Health of all Children Emergency Contraception Krishna K. Upadhya, MD, MPH, FAAP, COMMITTEE ON ADOLESCENCE Despite significant declines over the past 2 decades, the United States abstract continues to experience birth rates among teenagers that are significantly higher than other high-income nations. Use of emergency contraception (EC) within 120 hours after unprotected or underprotected intercourse can reduce the risk of pregnancy. Emergency contraceptive methods include oral Children’s National Health System, Washington, District of Columbia medications labeled and dedicated for use as EC by the US Food and Drug Policy statements from the American Academy of Pediatrics benefit Administration (ulipristal and levonorgestrel), the “off-label” use of combined from expertise and resources of liaisons and internal (AAP) and external reviewers. However, policy statements from the American oral contraceptives, and insertion of a copper intrauterine device. Indications Academy of Pediatrics may not reflect the views of the liaisons or the for the use of EC include intercourse without use of contraception; condom organizations or government agencies that they represent. breakage or slippage; missed or late doses of contraceptives, including the Dr Upadhya was responsible for all aspects of revising and writing the policy statement with input from reviewers and the Board of Directors; oral contraceptive pill, contraceptive patch, contraceptive ring, and injectable she approves the final manuscript as submitted. contraception; vomiting after use of oral contraceptives; and sexual assault. The guidance in this statement does not indicate an exclusive course Our aim in this updated policy statement is to (1) educate pediatricians and of treatment or serve as a standard of medical care. -
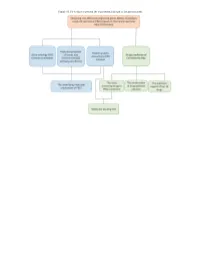
Figure S1. Flow Chart to Present the Experimental Design of the Present Study
Figure S1. Flow chart to present the experimental design of the present study. Figure S2. Volcano plot showing the differentially expressed genes between papillary renal cell carcinoma and non-cancer adjacent samples. Figure S3. The 2D molecular structures of the top 10 drugs. Table SI. The 60 potential drugs for papillary renal cell carcinoma treatment based on differently expressed genes. Drug name Dose Cell line Score Instance ID Pinacidil (C13H19N5) 16 µM HL60 -1 2406 Ciclosporin (C62H111N11O12) 3 µM HL60 -0.994 1331 Naftifine (C21H21N) 12 µM HL60 -0.966 2974 Vorinostat (C14H20N2O3) 10 µM HL60 -0.961 1161 Metacycline (C22H22N2O8) 8 µM HL60 -0.943 2901 Sulfacetamide (C8H10N2O3S) 16 µM HL60 -0.939 1859 Chlorprothixene (C18H18ClNS) 11 µM HL60 -0.938 1272 Amiodarone (C25H29I2NO3) 6 µM HL60 -0.933 2434 Noretynodrel (C20H26O2) 13 µM HL60 -0.914 1860 Valproic acid (C8H16O2) 200 µM HL60 -0.91 1155 Oxolinic acid (C13H11NO5) 15 µM HL60 -0.903 1419 Monocrotaline (C16H23NO6) 12 µM PC3 -0.881 7127 LY-294002 (C19H17NO3) 10 µM HL60 -0.869 1168 Aceclofenac (C16H13Cl2NO4) 11 µM PC3 -0.863 7269 Hyoscyamine (C17H23NO3) 14 µM HL60 -0.859 1424 Heliotrine (C16H27NO5) 13 µM HL60 -0.85 2180 Trichostatin A (C17H22N2O3) 1 µM HL60 -0.85 1175 Sulfachlorpyridazine (C10H8ClN4O2S) 14 µM HL60 -0.85 2191 Diflorasone (C22H28F2O5) 8 µM HL60 -0.838 2142 Yohimbine (C21H26N2O3) 10 µM MCF7 -0.833 6777 Sulfadiazine (C10H10N4O2S) 16 µM HL60 -0.833 1852 Pargyline (C11H13N) 20 µM PC3 -0.832 2102 Haloperidol (C21H23ClFNO2) 10 µM HL60 -0.829 6203 Methazolamide (C5H8N4O3S2) 17 -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0