Disseminated Intravascular Coagulation (DIC)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Crofab Brochure
Control With Confidence The only antivenom derived from native US pit vipers to treat envenomations from all species of North American pit vipers1 CroFab is the only antivenom Derived from geographically and clinically relevant US snakes for comprehensive coverage of all North American pit viper envenomations1 Designed with small, venom-specific protein (Fab) fragments for rapid neutralization of venom toxins throughout affected tissue1,2 With Level 1 evidence in the treatment of copperhead envenomation3 Manufactured to yield the highest level of quality, purity, and safety1 With a proven efficacy and safety profile, backed by >20 years of clinical experience1 Reliably supplied throughout the United States4 CroFab meets World Health Organization (WHO) guidelines for effective antivenom, utilizing venom from 4 clinically relevant pit viper species native to the United States.1,5 Indication CroFab® Crotalidae Polyvalent Immune Fab (Ovine) is a sheep-derived antivenin indicated for the management of adult and pediatric patients with North American crotalid envenomation. The term crotalid is used to describe the Crotalinae subfamily (formerly known as Crotalidae) of venomous snakes which includes rattlesnakes, copperheads and cottonmouths/water moccasins. Important Safety Information Contraindications Do not administer CroFab® to patients with a known history of hypersensitivity to any of its components, or to papaya or papain unless the benefits outweigh the risks and appropriate management for anaphylactic reactions is readily available. Warnings and Precautions Coagulopathy: In clinical trials, recurrent coagulopathy (the return of a coagulation abnormality after it has been successfully treated with antivenin), characterized by decreased fibrinogen, decreased platelets, and elevated prothrombin time, occurred in approximately half of the patients studied; one patient required re-hospitalization and additional antivenin administration. -
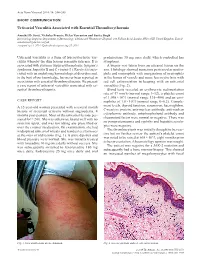
Urticarial Vasculitis Associated with Essential Thrombocythaemia
Acta Derm Venereol 2014; 94: 244–245 SHORT COMMUNICATION Urticarial Vasculitis Associated with Essential Thrombocythaemia Annabel D. Scott, Nicholas Francis, Helen Yarranton and Sarita Singh Dermatology Registrar, Department of Dermatology, Chelsea and Westminster Hospital, 369 Fulham Road, London SW10 9NH, United Kingdom. E-mail: [email protected] Accepted Apr 3, 2013; Epub ahead of print Aug 27, 2013 Urticarial vasculitis is a form of leucocytoclastic vas- prednisolone 30 mg once daily, which controlled her culitis whereby the skin lesions resemble urticaria. It is symptoms. associated with systemic lupus erythematosus, Sjögren’s A biopsy was taken from an uticarial lesion on the syndrome, hepatitis B and C viruses (1). Rarely it is asso- arm. Histology showed numerous perivascular neutro- ciated with an underlying haematological disorders and, phils and eosinophils with margination of neutrophils to the best of our knowledge, has never been reported in in the lumen of vessels and some leucocytoclasis with association with essential thrombocythaemia. We present red cell extravasation in keeping with an urticarial a case report of urticarial vasculitis associated with es- vasculitis (Fig. 2). sential thrombocythaemia. Blood tests revealed an erythrocyte sedimentation rate of 47 mm/h (normal range 1–12), a platelet count of 1,098 × 109/l (normal range 135–400) and an eosi- CASE REPORT nophilia of 1.0 × 109/l (normal range 0–0.2). Comple- A 32-year-old woman presented with a several month ment levels, thyroid function, serum iron, haemoglobin, history of recurrent urticaria without angioedema, 4 C-reactive protein, anti-nuclear antibody, anti-nuclear months post-partum. -

081999 Disseminated Intravascular Coagulation
The New England Journal of Medicine Current Concepts Systemic activation+ of coagulation DISSEMINATED INTRAVASCULAR COAGULATION Intravascular+ Depletion of platelets+ deposition of fibrin and coagulation factors MARCEL LEVI, M.D., AND HUGO TEN CATE, M.D. Thrombosis of small+ Bleeding and midsize vessels+ ISSEMINATED intravascular coagulation is and organ failure characterized by the widespread activation Dof coagulation, which results in the intravas- Figure 1. The Mechanism of Disseminated Intravascular Coag- cular formation of fibrin and ultimately thrombotic ulation. occlusion of small and midsize vessels.1-3 Intravascu- Systemic activation of coagulation leads to widespread intra- lar coagulation can also compromise the blood sup- vascular deposition of fibrin and depletion of platelets and co- agulation factors. As a result, thrombosis of small and midsize ply to organs and, in conjunction with hemodynam- vessels may occur, contributing to organ failure, and there may ic and metabolic derangements, may contribute to be severe bleeding. the failure of multiple organs. At the same time, the use and subsequent depletion of platelets and coag- ulation proteins resulting from the ongoing coagu- lation may induce severe bleeding (Fig. 1). Bleeding may be the presenting symptom in a patient with disseminated intravascular coagulation, a factor that can complicate decisions about treatment. TABLE 1. COMMON CLINICAL CONDITIONS ASSOCIATED WITH DISSEMINATED ASSOCIATED CLINICAL CONDITIONS INTRAVASCULAR COAGULATION. AND INCIDENCE Sepsis Infectious Disease Trauma Serious tissue injury Disseminated intravascular coagulation is an ac- Head injury Fat embolism quired disorder that occurs in a wide variety of clin- Cancer ical conditions, the most important of which are listed Myeloproliferative diseases in Table 1. -

What Everyone Should Know to Stop Bleeding After an Injury
What Everyone Should Know to Stop Bleeding After an Injury THE HARTFORD CONSENSUS The Joint Committee to Increase Survival from Active Shooter and Intentional Mass Casualty Events was convened by the American College of Surgeons in response to the growing number and severity of these events. The committee met in Hartford Connecticut and has produced a number of documents with rec- ommendations. The documents represent the consensus opinion of a multi-dis- ciplinary committee involving medical groups, the military, the National Security Council, Homeland Security, the FBI, law enforcement, fire rescue, and EMS. These recommendations have become known as the Hartford Consensus. The overarching principle of the Hartford Consensus is that no one should die from uncontrolled bleeding. The Hartford Consensus recommends that all citizens learn to stop bleeding. Further information about the Hartford Consensus and bleeding control can be found on the website: Bleedingcontrol.org 2 SAVE A LIFE: What Everyone Should Know to Stop Bleeding After an Injury Authors: Peter T. Pons, MD, FACEP Lenworth Jacobs, MD, MPH, FACS Acknowledgements: The authors acknowledge the contributions of Michael Cohen and James “Brooks” Hart, CMI to the design of this manual. Some images adapted from Adam Wehrle, EMT-P and NAEMT. © 2017 American College of Surgeons CONTENTS SECTION 1 3 ■ Introduction ■ Primary Principles of Trauma Care Response ■ The ABCs of Bleeding SECTION 2 5 ■ Ensure Your Own Safety SECTION 3 6 ■ A – Alert – call 9-1-1 SECTION 4 7 ■ B – Bleeding – find the bleeding injury SECTION 5 9 ■ C – Compress – apply pressure to stop the bleeding by: ■ Covering the wound with a clean cloth and applying pressure by pushing directly on it with both hands, OR ■Using a tourniquet, OR ■ Packing (stuff) the wound with gauze or a clean cloth and then applying pressure with both hands SECTION 6 13 ■ Summary 2 SECTION 1: INTRODUCTION Welcome to the Stop the Bleed: Bleeding Control for the Injured information booklet. -

Haemostatic Problems in Liver Disease
Gut: first published as 10.1136/gut.27.3.339 on 1 March 1986. Downloaded from Gut, 1986, 27, 339-349 Progress report Haemostatic problems in liver disease The liver plays a major role in the control of coagulation and as a result haemostatic problems are detected in approximately 75% of patients with liver disease.1 The coagulation abnormalities are both complex and multifactorial and depend on the balance between hepatic synthesis and clearance of activated coagulation proteins and their inhibitors; the presence or absence of dysfibrinogenaemia; thrombocytopenia, abnormal platelet function, and disseminated intravascular coagulation. Some patients will present with petechiae, ecchymosis or epistaxis, but most patients are asymptomatic or only bleed after venepuncture or liver biopsy. Alternatively haemorrhage may be life threatening and patients may die from variceal bleeding or from disseminated intravascular coagulation. The reasons for this disparity are not yet clear, but after the introduction of newer techniques, in particular the development of immunological assays for the antigens of coagulation proteins, our understanding of these problems has improved. The normal coagulation and fibrinolytic systems are depicted in Figures 1 and 2 while the major .__Intrinsic___ _ pathwY http://gut.bmj.com/ Kallikrein.o- PK | HMWKq 8t XII -*xiiXIIa_4------- ATIII ~ ~ 'I L1HMWK - XI* Xla %' xC-a; --------- -- on September 28, 2021 by guest. Protected copyright. IX - IXa VII -e'VIIca Extrinsic pathway [X VIII a Ce X P'okin C ATIII Ca+ XIII Common mI ~V PL II a pathway I XIIIa Fibrinogen - Fibrin Fig. 1 The coagulation cascade. HMWK=high molecular weight Kinogen, PK=Pre-Kallikrein, A TIII=antithrornbin III, PL=platelets, Ca" = Calcium, TF=tissue factor, -t- =proteolytic activation, -+=conversion ofcoagulation protein, -- -+=inhibition by plasma inhibitors, tit =crosslinking, a=activated coagulation enzyme. -

The Underrecognized Prothrombotic Vascular Disease of COVID-19
Journal Articles 2020 The underrecognized prothrombotic vascular disease of COVID-19. KP Cohoon G Mahé AC Spyropoulos Zucker School of Medicine at Hofstra/Northwell, [email protected] Follow this and additional works at: https://academicworks.medicine.hofstra.edu/articles Part of the Internal Medicine Commons Recommended Citation Cohoon K, Mahé G, Spyropoulos A. The underrecognized prothrombotic vascular disease of COVID-19.. 2020 Jan 01; 4(5):Article 6487 [ p.]. Available from: https://academicworks.medicine.hofstra.edu/articles/ 6487. Free full text article. This Article is brought to you for free and open access by Donald and Barbara Zucker School of Medicine Academic Works. It has been accepted for inclusion in Journal Articles by an authorized administrator of Donald and Barbara Zucker School of Medicine Academic Works. For more information, please contact [email protected]. Received: 6 May 2020 | Revised: 16 May 2020 | Accepted: 21 May 2020 DOI: 10.1002/rth2.12396 LETTER TO THE EDITOR The underrecognized prothrombotic vascular disease of COVID-19 We have read with interest “COVID-19-associated coagulopathy around elevated markers of hypercoagulability, including D-dimer, and thromboembolic disease: Commentary on an interim expert tissue factor expression, fibrinogen levels, factor VIII levels, guidance” recently provided by Cannegieter and Klok.1 This com- short-activated partial thromboplastin time, platelet binding, and mentary exemplifies the importance that venous thromboembolism thrombin formation.8 Based on well-defined clinical and laboratory (VTE) and atheroembolism may be underrepresented and a cause parameters, a proposal for staging COVID-19 coagulopathy may for increased morbidity and mortality among coronavirus disease provide treatment algorithms stratified into 3 stages.9 However, 2019 (COVID-19) patients. -
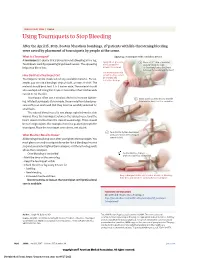
Using Tourniquets to Stop Bleeding
JAMA PATIENT PAGE | Trauma Using Tourniquets to Stop Bleeding After the April 15, 2013, Boston Marathon bombings, 27 patients with life-threatening bleeding were saved by placement of tourniquets by people at the scene. What Is a Tourniquet? Applying a tourniquet with a windlass device A tourniquet is a device that is placed around a bleeding arm or leg. Apply direct pressure 1 Place a 2-3” strip of material Tourniquets work by squeezing large blood vessels. The squeezing to the wound for about 2” from the edge helps stop blood loss. at least 15 minutes. of the wound over a long bone between the wound and the heart. Use a tourniquet only How Do I Put a Tourniquet On? when bleeding cannot be stopped and Tourniquets can be made out of any available material. For ex- is life threatening. ample, you can use a bandage, strip of cloth, or even a t-shirt. The material should be at least 2 to 3 inches wide. The material should also overlap itself. Using thin straps or material less than 2 inches wide can rip or cut the skin. Tourniquets often use a windlass device to increase tighten- 2 Insert a stick or other strong, straight ing. Inflated tourniquets (for example, those made from blood pres- item into the knot to act as a windlass. sure cuffs) can work well. But they must be carefully watched for small leaks. The injured blood vessel is not always right below the skin wound. Place the tourniquet between the injured vessel and the heart, about 2 inches from the closest wound edge. -

Guidelines for the Management of Haemophilia in Australia
Guidelines for the management of haemophilia in Australia A joint project between Australian Haemophilia Centre Directors’ Organisation, and the National Blood Authority, Australia © Australian Haemophilia Centre Directors’ Organisation, 2016. With the exception of any logos and registered trademarks, and where otherwise noted, all material presented in this document is provided under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Australia (http://creativecommons.org/licenses/by-nc-sa/3.0/au/) licence. You are free to copy, communicate and adapt the work for non-commercial purposes, as long as you attribute the authors and distribute any derivative work (i.e. new work based on this work) only under this licence. If you adapt this work in any way or include it in a collection, and publish, distribute or otherwise disseminate that adaptation or collection to the public, it should be attributed in the following way: This work is based on/includes the Australian Haemophilia Centre Directors’ Organisation’s Guidelines for the management of haemophilia in Australia, which is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Australia licence. Where this work is not modified or changed, it should be attributed in the following way: © Australian Haemophilia Centre Directors’ Organisation, 2016. ISBN: 978-09944061-6-3 (print) ISBN: 978-0-9944061-7-0 (electronic) For more information and to request permission to reproduce material: Australian Haemophilia Centre Directors’ Organisation 7 Dene Avenue Malvern East VIC 3145 Telephone: +61 3 9885 1777 Website: www.ahcdo.org.au Disclaimer This document is a general guide to appropriate practice, to be followed subject to the circumstances, clinician’s judgement and patient’s preferences in each individual case. -

ER Guide to Bleeding Disorders
Bleeding disorders ER guide to bleeding disorders 1 Table of contents 4 General Guidelines 4–5 national Hemophilia Foundation guidelines 5–10 Treatment options 10 HemopHilia a Name:__________________________________________________________________________________________________ 10–11 national Hemophilia Foundation guidelines Address:________________________________________________________________________________________________ 12 dosage chart Phone:__________________________________________________________________________________________________ 14–15 Treatment products 16 HemopHilia B In case of emergency, contact: ______________________________________________________________________________ 16 national Hemophilia Foundation guidelines Relation to patient:________________________________________________________________________________________ 17 dosage chart 18 Treatment products 19 HemopHilia a or B with inHiBiTors Diagnosis: Hemophilia A: Mild Moderate Severe 20 national Hemophilia Foundation guidelines Inhibitors Inhibitors Bethesda units (if known) ____________________________________ 21 Treatment products Hemophilia B: Mild Moderate Severe 22–23 Von willeBrand disease Inhibitors Inhibitors Bethesda units (if known) ____________________________________ 23–24 national Hemophilia Foundation guidelines von Willebrand disease: Type 1 Type 2 Type 3 Platelet type 25 Treatment products 27 Bibliography Preferred product:_________________________________________________________________________________________ Dose for life-threatening -

Appendix Search Strategy Treatment of Hemophilia.Pdf
Appendix Search strategies Hemophilia – general aspects PubMed (NLM) September 2009 Von Willebrand disease (TiAb) AND Controlled clinical trial (PT) NOT Purpura, Thrombocytopenic (Me) Angiohemophilia (TiAb) Meta analysis (PT) Blood coagulation disorders (Me) Randomized controlled trial (PT) Hemophilia (TiAb) Systematic (SB) Haemophilia (TiAb) Bleeding disorder (TiAb) Random* (Ti) Bleeding disorders (TiAb) OR Control* (Ti) NOT Medline (SB) ("controlled clinical trial"[Publication Type] OR "meta analysis"[Publication Type] OR "randomized controlled trial"[Publication Type] OR systematic[sb] OR ((random*[Title] OR control*[Title]) NOT Medline[sb])) AND ("von Willebrand Disease"[title/abstract] OR "angiohemophilia"[title/Abstract] OR "Blood Coagulation Disorders"[Mesh terms] OR "hemophilia"[title/abstract] OR "haemophilia"[title/abstract] OR "bleeding disorder"[title/abstract] OR "bleeding disorders"[Title/abstract]) NOT "Purpura, Thrombocytopenic"[MeSH Terms] 211 Hemophilia – general aspects Embase.com (Elsevier) September 2009 Blood clotting factor deficiency (Exp,MJR) AND Clinical trial (Exp) NOT Thrombocytopenic purpura (Exp) Von Willebrand disease (Ti) Intervention study (De) Angiohemophilia (Ti) Longitudinal study (De) Angiohaemophilia (Ti) Prospective study (De) Hemophilia (Ti) Meta analysis (De) Haemophilia (Ti) Systematic review (De) Bleeding disorder (Ti) Random* (Ti) Bleeding disorders (Ti) Control* (Ti) ('blood clotting factor deficiency'/exp/mjOR 'von willebrand disease':ti OR 'angiohemophilia':ti OR 'angiohaemophilia':ti OR 'hemophilia':ti -

Immune Thrombocytopenic Purpura with Subsequent Development of JAK2 V617F-Positive Essential Thrombocythemia: Case Report
ISSN: 2640-7914 DOI: https://dx.doi.org/10.17352/ahcrr CLINICAL GROUP Received: 13 July, 2021 Case Report Accepted: 03 August, 2021 Published: 04 August, 2021 *Corresponding author: Marisabel Hurtado-Castillo, Immune thrombocytopenic PGY-3, Internal Medicine, Department of Medicine at NYU, 536 Ovington Avenue Apartment 3, Brooklyn NYC 11209, USA, Tel: 718-312-9641; purpura with subsequent Email: Keywords: Immune thrombocytopenic purpura; development of JAK2 Essential thrombocythemia; JAK-STAT signaling path- way; JAK2(V617F) mutation V617F-positive essential https://www.peertechzpublications.com thrombocythemia: Case Report Marisabel Hurtado-Castillo1*, Brian Flaherty2 and Morris Jrada2 1PGY-3, Internal Medicine, Department of Medicine at NYU, Brooklyn, NY, USA 2Clinical Assistant Professor, Department of Medicine at NYU Grossman School of Medicine, Brooklyn, NY, USA Abstract The sequential occurrence of Immune Thrombocytopenic Purpura (ITP) and Essential Thrombocythemia (ET) has been reported in the literature on a few occasions, as these are two hematologic disorders with distinct etiologies and patients usually have contrasting clinical presentations. Our case highlights the sequential occurrence of ITP, followed by Janus kinase 2 (JAK2) (V617F)-positive ET in a 64-year-old white woman, after four years of follow-up. The pathophysiology relating to these two conditions is incompletely understood, however, JAK2(V617F) mutation has been found in all the cases reported. Early identifi cation of JAK2(V617F) mutation in a patient with a -

Thrombosis and Coagulopathy Guidance in COVID-19
Thrombosis and Coagulopathy Guidance in COVID-19 The risk of thrombosis in COVID-19 Patients with COVID-19 are at risk of venous thromboembolism (VTE), which is a deep vein thrombosis (DVT) or pulmonary embolism (PE). It is still unknown if this risk is higher in comparison to non-COVID acutely ill patients. How to interpret a D-dimer level in COVID-19 An elevated or rising D-dimer level is commonly seen in patients with COVID-19 (~50%) and is because of a profound inflammatory state. An elevated D-dimer alone does not warrant investigation for VTE unless there is also a high clinical suspicion for DVT and/or PE. Pulmonary embolism should be considered in admitted patients with COVID-19 who have unexplained worsening respiratory status/hypoxia, unexplained hypotension or tachycardia, or signs of DVT. If the D-dimer is normal, this has the ability to rule out VTE. Although the false negative rate of D-dimer testing (i.e. DVT/PE is present but the result is normal) is unknown in COVID-19 patients, low rates of 1- 2% using highly sensitive D-dimer assays have been reported in other high risk populations. Therefore, a normal level D-dimer level provides reasonable confidence that VTE is not present. Prevention of thrombosis All hospitalized patients with suspected or confirmed COVID-19 should receive pharmacologic thromboprophylaxis, preferably with low-molecular-weight heparin (LMWH). LMWH prophylaxis should be held if the patient is bleeding or has a platelet count <30 x 109/L. In patients where anticoagulation is contraindicated, use mechanical thromboprophylaxis (e.g.