BAT Annual Report and Form 20-F 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Report and Accounts 2019
IMPERIAL BRANDS PLC BRANDS IMPERIAL ANNUAL REPORT AND ACCOUNTS 2019 ACCOUNTS AND REPORT ANNUAL ANNUAL REPORT AND ACCOUNTS 2019 OUR PURPOSE WE CAN I OWN Our purpose is to create something Everything See it, seize it, is possible, make it happen better for the world’s smokers with together we win our portfolio of high quality next generation and tobacco products. In doing so we are transforming WE SURPRISE I AM our business and strengthening New thinking, My contribution new actions, counts, think free, our sustainability and value creation. exceed what’s speak free, act possible with integrity OUR VALUES Our values express who we are and WE ENJOY I ENGAGE capture the behaviours we expect Thrive on Listen, challenge, share, make from everyone who works for us. make it fun connections The following table constitutes our Non-Financial Information Statement in compliance with Sections 414CA and 414CB of the Companies Act 2006. The information listed is incorporated by cross-reference. Additional Non-Financial Information is also available on our website www.imperialbrands.com. Policies and standards which Information necessary to understand our business Page Reporting requirement govern our approach1 and its impact, policy due diligence and outcomes reference Environmental matters • Occupational health, safety and Environmental targets 21 environmental policy and framework • Sustainable tobacco programme International management systems 21 Climate and energy 21 Reducing waste 19 Sustainable tobacco supply 20 Supporting wood sustainability -

British American Tobacco's Submission to the WHO's
British American Tobacco’s submission to the WHO’s Framework Convention on Tobacco Control This is the submission of the British American Tobacco group of companies commenting on the WHO’s Framework Convention on Tobacco Control. We are the world’s most international tobacco group with an active presence in 180 countries. Our companies sell some of the world’s best known brands including Dunhill, Kent, State Express 555, Lucky Strike, Benson & Hedges, Rothmans and Pall Mall. Executive summary • The WHO’s proposed ‘Framework Convention on Tobacco Control’ is fundamentally flawed and will not achieve its objectives. • The tobacco industry, along with other industries involved in the manufacture and distribution of legal but risky products, is the subject of considerable public attention. It is important that the debate about tobacco remains open, objective, constructive and free from opportunistic criticism if we are effectively to address the real issues associated with tobacco. • British American Tobacco is responsible tobacco. We seek to operate in partnership with governments, who are significant stakeholders in our business, and other interested parties, based on our open acknowledgement that we make a risky product and therefore support sensible regulation. • British American Tobacco shares the World Health Organisation’s desire to reduce the health impact of tobacco use. This paper outlines British American Tobacco’s proposal for the sensible regulation of tobacco. • Our proposal will relieve the WHO of the cost and bureaucracy involved in its wish to become a single global tobacco regulator, leaving it free to do what it should be doing – policy orientation. Some facts about tobacco • Today over one billion adults, about one third of the world’s adult population, choose to smoke. -
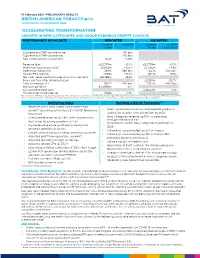
FY 2020 Announcement.Pdf
17 February 2021 –PRELIMINARY RESULTS BRITISH AMERICAN TOBACCO p.l.c. YEAR ENDED 31 DECEMBER 2020 ‘ACCELERATING TRANSFORMATION’ GROWTH IN NEW CATEGORIES AND GROUP EARNINGS DESPITE COVID-19 PERFORMANCE HIGHLIGHTS REPORTED ADJUSTED Current Vs 2019 Current Vs 2019 rates Rates (constant) Cigarette and THP volume share +30 bps Cigarette and THP value share +20 bps Non-Combustibles consumers1 13.5m +3.0m Revenue (£m) £25,776m -0.4% £25,776m +3.3% Profit from operations (£m) £9,962m +10.5% £11,365m +4.8% Operating margin (%) +38.6% +380 bps +44.1% +100 bps2 Diluted EPS (pence) 278.9p +12.0% 331.7p +5.5% Net cash generated from operating activities (£m) £9,786m +8.8% Free cash flow after dividends (£m) £2,550m +32.7% Cash conversion (%)2 98.2% -160 bps 103.0% +650 bps Borrowings3 (£m) £43,968m -3.1% Adjusted Net Debt (£m) £39,451m -5.3% Dividend per share (pence) 215.6p +2.5% The use of non-GAAP measures, including adjusting items and constant currencies, are further discussed on pages 48 to 53, with reconciliations from the most comparable IFRS measure provided. Note – 1. Internal estimate. 2. Movement in adjusted operating margin and operating cash conversion are provided at current rates. 3. Borrowings includes lease liabilities. Delivering today Building A Better TomorrowTM • Revenue, profit from operations and earnings • 1 growth* absorbing estimated 2.5% COVID-19 revenue 13.5m consumers of our non-combustible products , headwind adding 3m in 2020. On track to 50m by 2030 • New Categories revenue up 15%*, accelerating • Combustible revenue -

Companies Subject to Exclusion
Opdateret 01-08-2021 Vellivs Ekslusionsliste Velliv ønsker ikke at investere i selskaber, som bryder med Vellivs ESG-kriterier fastlagt i politikken for ansvarlig investering og aktivt ejerskab og som ikke udviser forandringsvilje i forhold til deres håndtering af ESG-risici. Endelig ønsker Velliv ikke at investere i tobaksselselskaber, selskaber med aktivteter i kontroversielle våben eller selskaber, hvor mere end 5 pct. af omsætningen stammer fra udvinding af kul og oliesand. Selsakb Kommentar 22nd Century Group, Inc. Tobacco AECOM Contr.Weapons Aerojet Rocketdyne Holdings, Inc. Contr.Weapons Aerojet Rocketdyne, Inc. Contr.Weapons Aeroteh SA Contr.Weapons Airbus SE Contr.Weapons Al-Eqbal Co. for Investment Plc Tobacco ALLETE, Inc. Kul Alliance Holdings GP LP Coal Alliance Resource Operating Partners LP Coal Alliance Resource Partners LP Coal Alpha Metallurgical Resources, Inc Coal Alpha Natural Resources Inc Coal Altadis Emisiones Financieras SA Tobacco Altadis SA Tobacco AltaGas Ltd. Gas/ESG Altius Minerals Corporation Coal Altria Group, Inc. Tobacco Anglo American plc Kul Anglo Pacific Group plc Coal Arch Resources, Inc. Coal Arcis Resources Corp. Tobacco Aryt Industries Ltd. Contr.Weapons Asenovgrad Tabac AD Tobacco Ashtrom Group Ltd. Normviolation>human rights Athabasca Oil Corporation Oilsand Avco Corp. Contr.Weapons B.A.T. Capital Corp. Tobacco B.A.T. Finance BV Tobacco B.A.T. International Finance Plc Tobacco Babcock International Group plc Contr.Weapons BADECO ADRIA dd Tobacco BAE Systems (Finance) Ltd. Contr.Weapons BAE Systems plc Contr.Weapons Bat Brasil Tobacco Bathurst Resources Limited Coal Baytex Energy (LP) Ltd. Oilsand Baytex Energy Corp. Oilsand Bellatora, Inc. Tobacco BHP Group Limited Kul BHP Group Plc Kul BlackPearl Resources, Inc. -

Vaping Lingo Dictionary
Vaping Lingo Dictionary High vaping rates among youth and young adults have introduced a plethora of new terms and keeping up with the latest lingo can be difficult. If you are wondering what the young people in your life are talking about, here is a list of some popular words, phrases, products and general language used to refer to vaping/e-cigarette use. POPULAR VAPE BRANDS & DEVICES Closed Pod System (uses disposable pods) JUUL BLU NJOY VUSE Vibe VUSE Alto Open/Refillable System Box Mod Suorin Drop Suorin Air NOVO Vape Juice Disposables BIDI Stick Cuvie (HQD) Puff Bars Posh Vape Stig POPULAR TERMS, PHRASES & SLANG USED BY YOUNG VAPERS The part that provides power to the heating element to warm Battery, Batt the e-liquid and produce vapor Blanks Empty cartridges a user can fill with the e-juice of their choice Cartridge, Cart A refillable vape juice container Used to recharge the e-cig battery once it has been depleted Charger Clone A knock-off of an original device that is typically less expensive A type of device that uses disposable pods containing e-liquid (typically ~200-500 puffs). The body of these devices can be recharged and the disposable pods can be replaced with new Closed Pod System compatible pods. What vapers call the vape mist that’s produced during vaping Clouds A Dab pen is used primarily for consuming THC concentrates and using the device is typically also referred to as “vaping.” These devices look and work much like other vape pens. Dab pen The “most prominent in a class of largely counterfeit brands, with common packaging that is easily available online and used by distributors to market THC-containing cartridges," according to a Centers for Disease Control and Prevention report on e- cigarette or vaping product use-associated lung injury. -

Annual Report 2013
Annual Report 2013 “ Being active and having a positive outlook on life is what keeps me going every day.” Overview of 2013 “ Our performance in 2013 was defined by remarkable &R D output and further delivery of sustained financial performance for our shareholders.” Please go to page 4 for more More at gsk.com Performance highlights £26.5bn £8.0bn £7.0bn £5.2bn Group turnover Core* operating profit Total operating profit Returned to shareholders 6 112.2p 112.5p 13% Major medicines approved Core* earnings per share Total earnings per share Estimated return on R&D investment 10 6 1st 1st Potential phase III study starts in 2014/15 Potential medicines with phase III data in Access to Medicines Index Pharmaceutical company to sign AllTrials expected 2014/15 campaign for research transparency Front cover story Betty, aged 65, (pictured) has Chronic “ Health is important to me, Obstructive Pulmonary Disease (COPD). She only has 25% lung capacity. This means I try to take care of my she finds even everyday tasks difficult, but medicines and inhaled oxygen allow her to health with all the tools live as normal a life as she can. Betty’s mindset I have and do the best is to stay busy and active, so every week she goes to rehab exercise classes. that I can with it.” COPD is a disease of the lungs that leads to Betty, COPD patient, damaged airways, causing them to become North Carolina, USA narrower and making it harder for air to get in and out. 210 million people around the world are estimated to have COPD. -

BAT INTERNATIONAL FINANCE Plc
BASE PROSPECTUS B.A.T. INTERNATIONAL FINANCE p.l.c. (incorporated with limited liability in England and Wales) BRITISH AMERICAN TOBACCO HOLDINGS (THE NETHERLANDS) B.V. (incorporated with limited liability in The Netherlands) B.A.T. NETHERLANDS FINANCE B.V. (incorporated with limited liability in The Netherlands) B.A.T CAPITAL CORPORATION (incorporated with limited liability in the State of Delaware, United States of America) £25,000,000,000 Euro Medium Term Note Programme unconditionally and irrevocably guaranteed by BRITISH AMERICAN TOBACCO p.l.c. (incorporated with limited liability in England and Wales) and each of the Issuers (except where it is the relevant Issuer) On 6 July 1998, each of B.A.T. International Finance p.l.c. (“BATIF”), B.A.T Capital Corporation (“BATCAP”) and B.A.T Finance B.V. (“BATFIN”) entered into a Euro Medium Term Note Programme (the “Programme”) for the issue of Euro Medium Term Notes (the “Notes”). On 16 April 2003, British American Tobacco Holdings (The Netherlands) B.V. (“BATHTN”) acceded to the Programme as an issuer and, where relevant, a guarantor and BATFIN was removed as an issuer and a guarantor under the Programme. On 9 December 2011, BATCAP was removed as an issuer and a guarantor under the Programme. On 16 May 2014, B.A.T. Netherlands Finance B.V. (“BATNF”) acceded to the Programme as an issuer and, where relevant, a guarantor. On 31 May 2017, BATCAP acceded to the Programme as an issuer and, where relevant, a guarantor. BATIF, BATHTN, BATNF and BATCAP are each, in their capacities as issuers under the Programme, an “Issuer” and together referred to as the “Issuers”. -

Imperial-Ham-La:Layout 1
CMAJ Special report Destroyed documents: uncovering the science that Imperial Tobacco Canada sought to conceal David Hammond MSc PhD, Michael Chaiton MSc, Alex Lee BSc, Neil Collishaw MA Previously published at www.cmaj.ca Abstract provided a wealth of information about the conduct of the tobacco industry, the health effects of smoking and the role Background: In 1992, British American Tobacco had its of cigarette design in promoting addiction.2 Canadian affiliate, Imperial Tobacco Canada, destroy inter- A number of the most sensitive documents were concealed nal research documents that could expose the company to or destroyed before the trial as the threat of litigation grew.3,4 liability or embarrassment. Sixty of these destroyed docu- Based on advice from their lawyers, companies such as ments were subsequently uncovered in British American British American Tobacco instituted a policy of document Tobacco’s files. destruction.5 A.G. Thomas, the head of Group Security at Methods: Legal counsel for Imperial Tobacco Canada pro- British American Tobacco, explained the criteria for selecting vided a list of 60 destroyed documents to British American reports for destruction: “In determining whether a redundant Tobacco. Information in this list was used to search for document contains sensitive information, holders should copies of the documents in British American Tobacco files released through court disclosure. We reviewed and sum- apply the rule of thumb of whether the contents would harm marized this information. or embarrass the Company or an individual if they were to be made public.”6 Results: Imperial Tobacco destroyed documents that British American Tobacco’s destruction policy was most included evidence from scientific reviews prepared by rigorously pursued by its subsidiaries in the United States, British American Tobacco’s researchers, as well as 47 ori - gin al research studies, 35 of which examined the biological Canada and Australia, likely because of the imminent threat activity and carcinogenicity of tobacco smoke. -

Sproule Et Al V. Santa Fe Natural Tobacco
Case 0:15-cv-62064-JAL Document 1 Entered on FLSD Docket 09/30/2015 Page 1 of 20 UNITED STATES DISTRICT COURT SOUTHERN DISTRICT OF FLORIDA JUSTIN SPROULE, individually and on behalf of all others similarly situated, No. Plaintiff, JURY TRIAL DEMANDED v. SANTA FE NATURAL TOBACCO COMPANY, INC., and REYNOLDS AMERICAN INC., Defendants. / CLASS ACTION COMPLAINT Plaintiff, Justin Sproule, individually, and on behalf of all others similarly situated in the United States, by and through the undersigned counsel, files this Class Action Complaint, and alleges against Defendants, Santa Fe Natural Tobacco Company, Inc. and Reynolds American Inc., as follows: INTRODUCTION 1. Defendants manufacture, market, and sell Natural American Spirit cigarettes (“American Spirits”). Defendants’ product labeling and advertising describes these cigarettes as “Natural,” “Additive Free,” “100% Additive Free,” “Organic,” and an “unadulterated tobacco product.”1 These terms are intended to suggest that American Spirits are healthier, safer, and present a lower risk of tobacco-related disease than other tobacco products. Defendants, however, have no competent or reliable scientific evidence to back their labeling and advertising claims. Defendants’ claims are patently deceptive, especially in today’s market, where these terms have a 1 https://www.sfntc.com/site/ourCompany/sfntc-story/ (last visited Aug. 28, 2015). Case 0:15-cv-62064-JAL Document 1 Entered on FLSD Docket 09/30/2015 Page 2 of 20 potent meaning for the health-and environmentally-conscious consumer. Moreover, as the FDA recently determined, American Spirits are in fact ‘adulterated.’ Using these deceptive terms, Defendants are able to successfully price American Spirits higher than other competitive cigarette brands. -

International Marketing Principles
British American Tobacco’s International Marketing Principles At British American Tobacco we believe in upholding high standards of corporate behaviour. We agree that the tobacco industry should be regulated, but we also think we should be able to communicate in a responsible way with adult tobacco consumers about our products, in order to grow market share. British American Tobacco’s International The Marketing Principles apply to the Marketing Principles provide a consistent marketing of all British American and responsible approach to marketing Tobacco’s combustible tobacco across the Group. They replace the products. International Marketing Standards which were launched in 2001 and updated in 2007. The Marketing Principles are our minimum The new Marketing Principles ensure our standard and will be applied even when they approach reflects developments in are stricter than local laws. However, if local marketing, technology and changing laws or other voluntary codes in markets are regulations and stakeholder expectations. stricter than or override our Marketing Principles, then we will abide by those laws The Marketing Principles comprise four core or voluntary codes. principles which we believe are at the heart of responsible tobacco marketing. The We will monitor and audit our performance rationale for each principle is explained and against the Marketing Principles and report illustrated by a set of core standards which our findings in the Group’s Sustainability show how they should be applied in our Report. The appendix at the end of this communications with consumers. document contains more information about the governance of the Marketing Principles. The Marketing Principles outline what all our Group Companies must consider when We expect all Group companies and anyone planning their tobacco marketing activities working on our behalf to adopt these but they do not offer standards for every Marketing Principles and seek to apply them activity. -

Annex 1: Parker Review Survey Results As at 2 November 2020
Annex 1: Parker Review survey results as at 2 November 2020 The data included in this table is a representation of the survey results as at 2 November 2020, which were self-declared by the FTSE 100 companies. As at March 2021, a further seven FTSE 100 companies have appointed directors from a minority ethnic group, effective in the early months of this year. These companies have been identified through an * in the table below. 3 3 4 4 2 2 Company Company 1 1 (source: BoardEx) Met Not Met Did Not Submit Data Respond Not Did Met Not Met Did Not Submit Data Respond Not Did 1 Admiral Group PLC a 27 Hargreaves Lansdown PLC a 2 Anglo American PLC a 28 Hikma Pharmaceuticals PLC a 3 Antofagasta PLC a 29 HSBC Holdings PLC a InterContinental Hotels 30 a 4 AstraZeneca PLC a Group PLC 5 Avast PLC a 31 Intermediate Capital Group PLC a 6 Aveva PLC a 32 Intertek Group PLC a 7 B&M European Value Retail S.A. a 33 J Sainsbury PLC a 8 Barclays PLC a 34 Johnson Matthey PLC a 9 Barratt Developments PLC a 35 Kingfisher PLC a 10 Berkeley Group Holdings PLC a 36 Legal & General Group PLC a 11 BHP Group PLC a 37 Lloyds Banking Group PLC a 12 BP PLC a 38 Melrose Industries PLC a 13 British American Tobacco PLC a 39 Mondi PLC a 14 British Land Company PLC a 40 National Grid PLC a 15 BT Group PLC a 41 NatWest Group PLC a 16 Bunzl PLC a 42 Ocado Group PLC a 17 Burberry Group PLC a 43 Pearson PLC a 18 Coca-Cola HBC AG a 44 Pennon Group PLC a 19 Compass Group PLC a 45 Phoenix Group Holdings PLC a 20 Diageo PLC a 46 Polymetal International PLC a 21 Experian PLC a 47 -

Imperial Tobacco Canada Limited and Imperial Tobacco Company Limited
Court File No. CV-19-616077-00CL Imperial Tobacco Canada Limited and Imperial Tobacco Company Limited PRE-FILING REPORT OF THE PROPOSED MONITOR March 12, 2019 TABLE OF CONTENTS GENERAL .....................................................................................................................................1 INTRODUCTION ............................................................................................................................1 BACKGROUND ..............................................................................................................................6 FORUM .........................................................................................................................................14 ACCOMMODATION AGREEMENT ................................................................................................15 THE TOBACCO CLAIMANT REPRESENTATIVE .............................................................................17 IMPERIAL’S CASH FLOW FORECAST ..........................................................................................18 COURT-ORDERED CHARGES .......................................................................................................20 CHAPTER 15 PROCEEDINGS ........................................................................................................23 RELIEF SOUGHT ...........................................................................................................................24 CONCLUSION ...............................................................................................................................25