COVID-19: Neurological Considerations in Neonates and Children
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Management and Investigation of Neonatal Encephalopathy: 2017 Update Kathryn Martinello,1 Anthony R Hart,2 Sufin Yap,3 Subhabrata Mitra,1 Nicola J Robertson1
Review Arch Dis Child Fetal Neonatal Ed: first published as 10.1136/archdischild-2015-309639 on 6 April 2017. Downloaded from Management and investigation of neonatal encephalopathy: 2017 update Kathryn Martinello,1 Anthony R Hart,2 Sufin Yap,3 Subhabrata Mitra,1 Nicola J Robertson1 1Department of Neonatology, ABSTRACT definite aetiological diagnosis is known, and Institute for Women’s Health, This review discusses an approach to determining the hypoxic-ischaemic encephalopathy (HIE) where University College London, UK 2 cause of neonatal encephalopathy, as well as current clear diagnosis of hypoxia-ischaemia is known to Department of Neonatal and ’ Paediatric Neurology, Sheffield evidence on resuscitation and subsequent management have led to the neonate s clinical state. Children’s Hospital NHS of hypoxic-ischaemic encephalopathy (HIE). Foundation Trust, Sheffield, UK Encephalopathy in neonates can be due to varied 3 DETERMINING THE AETIOLOGY OF NE Department of Inherited aetiologies in addition to hypoxic-ischaemia. A Metabolic Diseases, Sheffield The initial stages of managing NE will be the same Children’s Hospital NHS combination of careful history, examination and the for most babies, with good resuscitation and sup- Foundation Trust, Sheffield, UK judicious use of investigations can help determine the portive management. However, as the picture cause. Over the last 7 years, infants with moderate to evolves and investigations return, clinicians should fi Correspondence to severe HIE have bene ted from the introduction of consider the aetiology of NE as this could lead to Professor Nicola J Robertson, routine therapeutic hypothermia; the number needed to specific treatments, aid with prognosis and recur- Institute for Women’s Health, treat for an additional beneficial outcome is 7 (95% CI University College London, 74 rence risk counselling, and assist with the evalu- Huntley Street, London WC1E 5 to 10). -

Hypotonia and Lethargy in a Two-Day-Old Male Infant Adrienne H
Hypotonia and Lethargy in a Two-Day-Old Male Infant Adrienne H. Long, MD, PhD,a,b Jennifer G. Fiore, MD,a,b Riaz Gillani, MD,a,b Laurie M. Douglass, MD,c Alan M. Fujii, MD,d Jodi D. Hoffman, MDe A 2-day old term male infant was found to be hypotonic and minimally abstract reactive during routine nursing care in the newborn nursery. At 40 hours of life, he was hypoglycemic and had intermittent desaturations to 70%. His mother had an unremarkable pregnancy and spontaneous vaginal delivery. The mother’s prenatal serology results were negative for infectious risk factors. Apgar scores were 9 at 1 and 5 minutes of life. On day 1 of life, he fed, stooled, and voided well. Our expert panel discusses the differential diagnosis of hypotonia in a neonate, offers diagnostic and management recommendations, and discusses the final diagnosis. DRS LONG, FIORE, AND GILLANI, birth weight was 3.4 kg (56th PEDIATRIC RESIDENTS percentile), length was 52 cm (87th aDepartment of Medicine, Boston Children’s Hospital, d e percentile), and head circumference Boston, Massachusetts; and Neonatology Section, Medical A 2-day old male infant born at Genetics Section, cDivision of Child Neurology, and 38 weeks and 4 days was found to be was 33 cm (12th percentile). His bDepartment of Pediatrics, Boston Medical Center, Boston, limp and minimally reactive during physical examination at birth was Massachusetts routine care in the newborn nursery. normal for gestational age, with Drs Long, Fiore, and Gillani conceptualized, drafted, Just 5 hours before, he had an appropriate neurologic, cardiac, and and edited the manuscript; Drs Douglass, Fujii, and appropriate neurologic status when respiratory components. -

Hypotonia Surestep Product Catalog Page 29 in Step with Pediatric Hypotonia
SPECIAL EDUCATIONAL SERIES DIAGNOSTIC INSIGHTS ANALYZING GAIT CHANGES GROSS MOTOR SKILLS ORTHOTIC MANAGEMENT CLI N I CAL CASE STUDIES Sponsored by an educational grant from: In Step With Pediatric Hypotonia SureStep Product Catalog Page 29 In Step With Pediatric Hypotonia Contents VIEWPOINT FROM THE EDITOR: An Unexpected Path, Mobility and More an Invaluable Perspective At the most basic level, mobility is about get- PAGE 3 ting from point A to point B. But, for many children with hypotonia, it’s about so much 4 more. FEATURES It’s about independence. It’s about con- fidence. It’s about maintaining strength, fit- ness, and healthy bones. It’s about not being Understanding Hypotonia excluded from activities enjoyed by their PAGE 4 typically developing peers. And improved mobility may have even Gait: The Cornerstone more benefits in those children whose hy- potonia is associated with social and behav- of Intervention ioral developmental delays. New research PAGE 8 has identified an association between motor skills and sociobehavioral milestones in chil- 8 The Importance of Gross dren with autism spectrum disorder, who often present with hypotonia (see “The Im- Motor Skills portance of Gross Motor Skills,” page 12). PAGE 12 This suggests that early intervention to improve gross motor skills—including or- thotic devices and physical therapy—may Orthotic Solutions for also help certain children interact more Children with Hypotonia comfortably with others. That won’t come as PAGE 16 a surprise to the clinicians and parents who 12 have personally seen it happen. This special issue is filled with evidence- Orthotic Success Stories: based information and personal success sto- Four Cases in a Series ries illustrating how effective interventions can enhance mobility in children with hy- PAGE 20 potonia. -

Various Outcomes of Idiopathic Grade IV Intraventricular Haemorrhage in Term Newborns at Two Years of Age. Das S1, Bhattacharya M2, Chatterjee K3, Sarkar N4, Aich B5
Bangladesh Journal of Medical Science Vol. 17 No. 02 April’18 Case report: Various outcomes of Idiopathic Grade IV Intraventricular Haemorrhage in term newborns at two years of age. Das S1, Bhattacharya M2, Chatterjee K3, Sarkar N4, Aich B5 Bangladesh Journal of Medical Science Vol. 17 No. 02 April’18. Page : 316-318 DOI: http://dx.doi.org/10.3329/bjms.v17i2.35893 Introduction: duration of NICU stay was 15 days. Intraventricular Haemorrhage (IVH) generally Case1 (corresponds to Figure1) - At 2 years of age, occurs in infants <32 weeks and/or <1500 grams. he was developing right sided spastic hemiparetic Incidence of IVH in term neonates is 3.5-5% 1,2 . cerebral palsy. Right sided limbs exhibited 50% of IVH in term neonates is primarily caused by hypertonia, brisk deep tendon reflexes, ankle clonus trauma and asphyxia; a minority of haemorrhages and persistence of cortical thumb. The child showed is caused by extension of bleed from Subdural, early hand preference, dwarfing and dyspraxia of Subarachnoid and Intraparenchymal haemorrhage affected limbs. Electroencephalogram (EEG), Visual or caused by vascular lesions, coagulopathies or Evoked Potential (VEP), Brainstem Auditory Evoked tumours. 25% of cases have no significant risk Response (BAER) and Fundoscopy were normal factors. Most of the germinal matrix has regressed at 2 years of age. Developmental assesment was by term, so most haemorrhages (35%) arise from the done by Developmental Assesment Scale For Indian posterior tufts at the glomus in choroid plexus, 24% Infants (DASII) which is based on Bayley Scale of from Thalamus, 17% from residual Germinal Matrix Infant Development (BSID) II norms. -

Mid-Trimester Preterm Premature Rupture of Membranes (PPROM): Etiology, Diagnosis, Classification, International Recommendations of Treatment Options and Outcome
J. Perinat. Med. 2018; 46(5): 465–488 Review article Open Access Michael Tchirikov*, Natalia Schlabritz-Loutsevitch, James Maher, Jörg Buchmann, Yuri Naberezhnev, Andreas S. Winarno and Gregor Seliger Mid-trimester preterm premature rupture of membranes (PPROM): etiology, diagnosis, classification, international recommendations of treatment options and outcome DOI 10.1515/jpm-2017-0027 neonates delivered without antecedent PPROM. The “high Received January 23, 2017. Accepted May 19, 2017. Previously pub- PPROM” syndrome is defined as a defect of the chorio- lished online July 15, 2017. amniotic membranes, which is not located over the inter- nal cervical os. It may be associated with either a normal Abstract: Mid-trimester preterm premature rupture of mem- or reduced amount of amniotic fluid. It may explain why branes (PPROM), defined as rupture of fetal membranes sensitive biochemical tests such as the Amniosure (PAMG-1) prior to 28 weeks of gestation, complicates approximately or IGFBP-1/alpha fetoprotein test can have a positive result 0.4%–0.7% of all pregnancies. This condition is associ- without other signs of overt ROM such as fluid leakage with ated with a very high neonatal mortality rate as well as an Valsalva. The membrane defect following fetoscopy also increased risk of long- and short-term severe neonatal mor- fulfils the criteria for “high PPROM” syndrome. In some bidity. The causes of the mid-trimester PPROM are multi- cases, the rupture of only one membrane – either the cho- factorial. Altered membrane morphology including marked rionic or amniotic membrane, resulting in “pre-PPROM” swelling and disruption of the collagen network which is could precede “classic PPROM” or “high PPROM”. -
IUGR: Intrauterine Growth Restriction
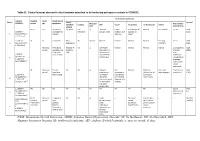
Table S1. Clinical features observed in the 6 patients described so far harboring pathogenic variants in FOXRED1. Evolutionary symptoms Variants Prenatal Onset Onset clinical Patient Lactic/ Survival FOXRED1 period age symptoms Muscular Psychomotor Metabolic Epilepsy MRI Visual Respiratory Cardiovascular Others tone development acidosis IUGR 2m Hypotonia Yes (+++) Yes ↓ Normal Latent Bronchospasm Normal AEP normal IQ: 48 Alive c.920G>A Development refractary (2m,4y,7y3m) strabismus of episodes in (15y) 1 (p.Gly307Glu) / al delay right eye infant c.733+1G>A c.920G>A NI 4y Clumsiness With No Normal Normal Normal Normal Normal Learning IQ: 99 Alive 2 (p.Gly307Glu) / exercise (+) disorders (19y) c.733+1G>A - Neonatal Premature; No (only ↑ Yes ↓ Decreased Normal Normal Normal Normal Gradually loss Alive period Hypoglycemia lactate in attenuation in of motor (22y)) Congenital LCR) the putamen skills; c.694C>T lactic acidosis and cerebellar wheelchair; (p.Gln232X) / 3 atrophy (6y) no expressive c.1289A>G language; 18 (p.Asn430Ser) understands simple commands NI Neonatal Truncal Yes Yes ↓ Delayed Eye Normal Mild non- Persistent Psychomotor Alive period hypotonia myelination movements obstructive left hepatomegaly retardation (10y) c.1054 C>T Poor feeding ventricular have always ventricular (p.Arg352Trp) / dilatation; been roving hypertrophy 4 c.1054 C>T abnormal signal bilateral optic (p.Arg352Trp) 19 in the thalami atrophy and basal ganglia (8m) c.1308G>A ND ND ND ND Yes NA NA NA NA NA NA Severe Alive (p.Val421Met) / psychomotor (¿) 5 c.1308G>A retardation (p.Val421Met)20 IUGR; Neonatal Congenital Yes Yes ↓ Large -- Persistent Dilated right - - Death (3 c.612_615dupA Cerebral period lactic periventricular severe ventricle and months) CTG intraventric acidosis. -

Cerebral Hypotonia by Mihee Bay MD (Dr
Cerebral hypotonia By Mihee Bay MD (Dr. Bay of Kennedy Krieger Institute and Johns Hopkins School of Medicine has no relevant financial relationships to disclose.) Originally released July 12, 2006; last updated February 1, 2016; expires February 1, 2019 Introduction This article includes discussion of cerebral hypotonia, central hypotonia, essential hypotonia, benign congenital hypotonia, and floppy infant. The foregoing terms may include synonyms, similar disorders, variations in usage, and abbreviations. Overview Hypotonia is a clinical manifestation of numerous diseases affecting the central and/or peripheral motor nervous system. The key to accurate diagnosis involves integral steps of evaluation that include a detailed history, examination, and diagnostic tests. “Cerebral” (or central) hypotonia implies pathogenesis from abnormalities from the central nervous system, and related causal disorders include cerebral dysgenesis and genetic or metabolic disorders. Patients with central hypotonia generally have hypotonia without associated weakness, in contrast to the peripheral (lower motor neuron) causes, which typically produce both hypotonia and muscle weakness. Hypotonia is a clinical manifestation of over 500 genetic disorders; thus, a logical, stepwise approach to diagnosis is essential. With recent advances in the field of genetic testing, diagnostic yield will undoubtedly improve. There is no cure, but treatment includes supportive therapies, such as physical and occupational therapy, and diagnosis-specific management. Key points • Hypotonia is reduced tension or resistance of passive range of motion. • The first step in the evaluation of a child with hypotonia is localization to the central (“cerebral”) or peripheral nervous system, or both. • Central hypotonia is more likely to be noted axially with normal strength and hyperactive to normal deep tendon reflexes. -

Hyperbilirubinemia and Kernicterus Jesus Peinado PGY2 Merle Ipson MD March 2009 Hyperbilirubinemia
Hyperbilirubinemia and Kernicterus Jesus Peinado PGY2 Merle Ipson MD March 2009 Hyperbilirubinemia Most common clinical condition requiring evaluation and treatment in the NB Most common cause of readmission in the 1 st week Generally a benign transitional phenomenon May pose a direct threat of brain damage May evolve into kernicterus Kernicterus 1. Choreoathetoid cerebral palsy 2. High-frequency central neural hearing loss 3. Palsy of vertical gaze 4. Dental enamel hypoplasia (result of bilirubin-induced cell toxicity) Kernicterus Originally described in NB with Rh hemolytic disease Recently reported in healthy term and late preterm Reported in breast-fed infants w/out hemolysis Most prevalent risk factor is late preterm Late Preterm Infant Relatively immature in their capacity to handle unconjugated bilirubin Hyperbilirubinemia is more prevalent, pronounced and protracted Eightfold increased risk of developing TSB > 20 mg/dl (5.2%) compared to term (0.7%) Pathobiology Increased bilirubin load in the hepatocyte Decreased erythrocyte survival Increased erythrocyte volume Increased enterohepatic circulation Decreased hepatic uptake from plasma Defective bilirubin conjugation Bilirubin Metabolism Bilirubin Metabolism Bilirubin Metabolism How bilirubin Damages the Brain Determinants of neuronal injury by bilirubin 1. Concentration of unconjugated bilirubin 2. Free bilirubin 3. Concentration of serum albumin 4. Ability to bind UCB 5. Concentration of hydrogen ion 6. Neuronal susceptibility Intracellular Calcium Homeostasis -

Mean Platelet Volume in Asymptomatic Chorioamnionitis-Exposed Infants
www.jpnim.com Open Access eISSN: 2281-0692 Journal of Pediatric and Neonatal Individualized Medicine 2021;10(1):e100132 doi: 10.7363/100132 Received: 2019 Aug 22; revised: 2020 Jan 26; accepted: 2020 Feb 02; published online: 2020 Dec 28 Original article Mean platelet volume in asymptomatic chorioamnionitis- exposed infants. A retrospective case-control study Atef Alshafei, Moustafa Hassan, Yaser El saba, Anwar Khan, Mahmoud Ahmed Neonatology Section, Pediatric Department, Dubai Hospital, Dubai, UAE Abstract Introduction: Maternal chorioamnionitis (CA) is a serious condition causing several neonatal morbidities and long-term neurodevelopmental sequelae in exposed infants. Current guidelines still recommend admission, laboratory evaluation, and antibiotic administration to all CA-exposed infants. The incidence of early-onset neonatal sepsis (EOS) is currently low, owing to the routine intrapartum antibiotic administration to mothers identified to be at risk of developing CA. New diagnostic tools for early diagnosis of sepsis in apparently healthy infants exposed to maternal CA are needed. Previous studies showed that mean platelet volume (MPV) is evolving as a potential inflammatory marker of neonatal sepsis. We aimed to study whether MPV can be used as an adjuvant diagnostic tool for EOS in asymptomatic CA-exposed infants. Objective: To evaluate the role of MPV as an adjuvant biomarker of EOS in cases of asymptomatic CA-exposed infants. Design: Retrospective case-control study. Setting: A tertiary care Neonatal Intensive Care Unit (NICU). Patients: Asymptomatic CA-exposed infants 37-40 weeks of gestation admitted between May 2016 and April 2019 to the NICU of Dubai Hospital, UAE. Results: A total of 1,300 infants were admitted to NICU during the study period. -

Long-Term Outcome After Neonatal Meconium Obstruction
Long-term Outcome After Neonatal Meconium Obstruction Julie R. Fuchs, MD, and Jacob C. Langer, MD ABSTRACT. Objective. It is unclear whether children meconium ileus and those undergoing resection or enter- with cystic fibrosis (CF) who present with neonatal ostomy. Patients with meconium obstruction who do not meconium ileus have a different long-term outcome from have CF have an excellent long-term prognosis. This those presenting later in childhood with pulmonary com- information will be useful in counseling the families of plications or failure to thrive. We examined a cohort of infants presenting with neonatal meconium obstruction. patients with meconium ileus, and compared their long- Pediatrics 1998;101(4). URL: http://www.pediatrics.org/ term outcome with children who had CF without meco- cgi/content/full/101/4/e7; cystic fibrosis, meconium ileus, nium ileus and neonates who had meconium obstruction meconium plug syndrome. without CF (meconium plug syndrome). Study Design. Comparative study using retrospective and follow-up interview data. ABBREVIATION. CF, cystic fibrosis. Patients. Group 1 consisted of 35 surviving CF pa- tients who presented with meconium ileus between 1966 econium obstruction in the neonate is a and 1992. Two control groups were also studied: 35 age- spectrum of disease that includes meco- and sex-matched CF patients without meconium ileus 1 (group 2), and 12 infants presenting with meconium plug Mnium ileus and meconium plug syndrome. syndrome during the same time period (group 3). Meconium ileus is characterized by extremely viscid, Outcome Measures. Pulmonary, gastrointestinal, nu- protein-rich inspissated meconium causing terminal tritional, and functional status were reviewed, and sur- ileal obstruction, and accounts for approximately gical complications were recorded. -

Subset of Alphabetical Index to Diseases and Nature of Injury for Use with Perinatal Conditions (P00-P96)
Subset of alphabetical index to diseases and nature of injury for use with perinatal conditions (P00-P96) SUBSET OF ALPHABETICAL INDEX TO DISEASES AND NATURE OF INJURY FOR USE WITH PERINATAL CONDITIONS (P00-P96) Conditions arising in the perinatal period Conditions arising—continued - abnormal, abnormality—continued Note - Conditions arising in the perinatal - - fetus, fetal period, even though death or morbidity - - - causing disproportion occurs later, should, as far as possible, be - - - - affecting fetus or newborn P03.1 coded to chapter XVI, which takes - - forces of labor precedence over chapters containing codes - - - affecting fetus or newborn P03.6 for diseases by their anatomical site. - - labor NEC - - - affecting fetus or newborn P03.6 These exclude: - - membranes (fetal) Congenital malformations, deformations - - - affecting fetus or newborn P02.9 and chromosomal abnormalities - - - specified type NEC, affecting fetus or (Q00-Q99) newborn P02.8 Endocrine, nutritional and metabolic - - organs or tissues of maternal pelvis diseases (E00-E99) - - - in pregnancy or childbirth Injury, poisoning and certain other - - - - affecting fetus or newborn P03.8 consequences of external causes (S00-T99) - - - - causing obstructed labor Neoplasms (C00-D48) - - - - - affecting fetus or newborn P03.1 Tetanus neonatorum (A33) - - parturition - - - affecting fetus or newborn P03.9 - ablatio, ablation - - presentation (fetus) (see also Presentation, - - placentae (see also Abruptio placentae) fetal, abnormal) - - - affecting fetus or newborn -

Motor Deficits Are Triggered by Reperfusion-Reoxygenation Injury As Diagnosed by MRI and by a Mechanism Involving Oxidants
5500 • The Journal of Neuroscience, April 18, 2012 • 32(16):5500–5509 Neurobiology of Disease Motor Deficits Are Triggered by Reperfusion-Reoxygenation Injury as Diagnosed by MRI and by a Mechanism Involving Oxidants Alexander Drobyshevsky,1 Kehuan Luo,1 Matthew Derrick,1 Lei Yu,1 Hongyan Du,2 P. V. Prasad,3 Jeannette Vasquez-Vivar,4 Ines Batinic-Haberle,5 and Sidhartha Tan1 1Department of Pediatrics, 2Center on Outcomes, Research and Education, and 3Radiology, NorthShore University Healthcare Research Institute, Evanston, Illinois 60201, 4Department of Biophysics, Medical College of Wisconsin, Milwaukee, Wisconsin 53226, and 5Department of Radiation Oncology, Duke University Medical Center, Durham, North Carolina 27710 The early antecedents of cerebral palsy (CP) are unknown but are suspected to be due to hypoxia-ischemia (H-I). In our rabbit model of CP, the MRI biomarker, apparent diffusion coefficient (ADC) on diffusion-weighted imaging, predicted which fetuses will develop postnatalhypertonia.SurvivingH-Ifetusesexperiencereperfusion-reoxygenationbutasubpopulationmanifestedacontinueddeclineof ADC during early reperfusion-reoxygenation, which possibly represented greater brain injury (RepReOx). We hypothesized that oxida- tive stress in reperfusion-reoxygenation is a critical trigger for postnatal hypertonia. We investigated whether RepReOx predicted postnatal neurobehavior, indicated oxidative stress, and whether targeting antioxidants at RepReOx ameliorated motor deficits, which included testing of a new superoxide dismutase mimic (MnTnHex-2-PyP). Rabbit dams, 79% gestation (E25), were subjected to 40 min uterine ischemia. Fetal brain ADC was followed during H-I, immediate reperfusion-reoxygenation, and 4–72 h after H-I. Endpoints were postnatal neurological outcome at E32, ADC at end of H-I, ADC nadir during H-I and reperfusion-reoxygenation, and area under ADC curve during the first 20 min of reperfusion-reoxygenation.