UNITED STATES' PATENT Ornron
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Billing Code: 3510-Ds-P Department Of
This document is scheduled to be published in the Federal Register on 01/30/2015 and available online at http://federalregister.gov/a/2015-01795, and on FDsys.gov BILLING CODE: 3510-DS-P DEPARTMENT OF COMMERCE International Trade Administration C-570-009 Calcium Hypochlorite from the People’s Republic of China: Countervailing Duty Order AGENCY: Enforcement and Compliance, International Trade Administration, Department of Commerce SUMMARY: Based on affirmative final determinations by the Department of Commerce (“Department”) and the International Trade Commission (“ITC”), the Department is issuing a countervailing duty order on calcium hypochlorite from the People’s Republic of China (“PRC”). EFFECTIVE DATE: [(Insert date of publication in the Federal Register]. FOR FURTHER INFORMATION CONTACT: Katie Marksberry, AD/CVD Operations, Office V, Enforcement and Compliance, International Trade Administration, U.S. Department of Commerce, 14th Street and Constitution Avenue, NW, Washington, DC 20230; telephone: (202) 482-7906. SUPPLEMENTARY INFORMATION: Background In accordance with section 705(d) of the Tariff Act of 1930, as amended (“the Act”), on December 15, 2014, the Department published its final determination that countervailable subsidies are being provided to producers and exporters of calcium hypochlorite from the PRC.1 On January 21, 2015, the ITC notified the Department of its final determination pursuant to 1 See Calcium Hypochlorite from the People’s Republic of China: Final Affirmative Countervailing Duty Determination; 79 FR -

THE JOURNAL of INDUSTRIAL and ENGINEERING CHEMISTRY 953 with Stirring, and the Flask Allowed to Stand
OCt., 1917 THE JOURNAL OF INDUSTRIAL AND ENGINEERING CHEMISTRY 953 with stirring, and the flask allowed to stand. The ascertaining the presence of aniline, the calcium hypo- solution, when the precipitate has settled, is filtered chlorite test is employed. The quantitative deter- through a Gooch crucible, using a bell jar arrangement, mination is carried out by precipitating the aniline into a 250 to 300 cc. Erlenmeyer flask. The crucible as tribromoaniline with bromine water. The precipi- is fitted preferably with a small circular filter paper tate is caught on a tared filter paper, dried in vacuo, (cut to size) instead of asbestos. The flask should be and weighed. washed with wash-ether (100parts absolute ether and In this connection, it occurred to the writer that it 2 parts alcoholic HC1) while filtering so as not to allow might be possible to work out, for estimating the any ferric chloride to dry on the white precipitate or aniline, a colorimetric method based on the qualita- on the crucible. The usual precautions of washing the tive test with hypochlorite. If this were possible, flask, crucible and funnel are taken to insure complete it certainly would be desirable, for not only would transfer of iron. It is not necessary, however, to trans- considerable time and effort be saved but probably fer all of the aluminum chloride precipitate to the a considerably smaller sample than IO liters would be Gooch crucible. The aluminum precipitate is removed sufficient and quantitative measurements could also with the paper from the crucible by tapping it into a be applied in cases where the amount of aniline in 25 cc. -

Chloroform 18.08.2020.Pdf
Chloroform Chloroform, or trichloromethane, is an organic compound with formula CHCl3. It is a colorless, sweet-smelling, dense liquid that is produced on a large scale as a precursor to PTFE. It is also a precursor to various refrigerants. It is one of the four chloromethanes and a trihalomethane. It is a powerful anesthetic, euphoriant, anxiolytic and sedative when inhaled or ingested. Formula: CHCl₃ IUPAC ID: Trichloromethane Molar mass: 119.38 g/mol Boiling point: 61.2 °C Density: 1.49 g/cm³ Melting point: -63.5 °C The molecule adopts a tetrahedral molecular geometry with C3v symmetry. Chloroform volatilizes readily from soil and surface water and undergoes degradation in air to produce phosgene, dichloromethane, formyl chloride, carbon monoxide, carbon dioxide, and hydrogen chloride. Its half-life in air ranges from 55 to 620 days. Biodegradation in water and soil is slow. Chloroform does not significantly bioaccumulate in aquatic organisms. Production:- In industry production, chloroform is produced by heating a mixture of chlorine and either chloromethane (CH3Cl) or methane (CH4). At 400–500 °C, a free radical halogenation occurs, converting these precursors to progressively more chlorinated compounds: CH4 + Cl2 → CH3Cl + HCl CH3Cl + Cl2 → CH2Cl2 + HCl CH2Cl2 + Cl2 → CHCl3 + HCl Chloroform undergoes further chlorination to yield carbon tetrachloride (CCl4): CHCl3 + Cl2 → CCl4 + HCl The output of this process is a mixture of the four chloromethanes (chloromethane, dichloromethane, chloroform, and carbon tetrachloride), which can then be separated by distillation. Chloroform may also be produced on a small scale via the haloform reaction between acetone and sodium hypochlorite: 3 NaClO + (CH3)2CO → CHCl3 + 2 NaOH + CH3COONa Deuterochloroform[ Deuterated chloroform is an isotopologue of chloroform with a single deuterium atom. -

CHEMICAL STORAGE SEGREGATION GUIDELINES Incompatible Chemicals Should Always Be Handled and Stored So That They Do Not Accidentally Come in Contact with Each Other
Laboratory Safety Reminders January 2007 ♦ Mount Holyoke College – Environmental Health and Safety CHEMICAL STORAGE SEGREGATION GUIDELINES Incompatible chemicals should always be handled and stored so that they do not accidentally come in contact with each other. This list is not complete, nor are all compatibilities shown. These materials can react to produce excessive heat, harmful vapors, and/or other deadly reactions. Always know the hazards and incompatibilities of a chemical before using it. Chemicals Avoid Accidental Contact With Acetic acid Chromic acid, nitric acid, permanganates, peroxides Hydroxyl-containing compounds such as perchloric acid, Acetic anhydride ethylene glycol Concentrated nitric acid and sulfuric acid mixtures, peroxides (i.e. Acetone peracetic acid solution, hydrogen peroxide) Acetylene Chlorine, bromine, copper, silver, fluorine, mercury Alkali, alkaline earth and strongly electropositive metals (powered Carbon dioxide, carbon tetrachloride and other chlorinated aluminum, magnesium, sodium, hydrocarbons potassium) Mercury, chlorine, calcium hypochlorite, iodine, bromine, hydrogen Ammonia (anhydrous) fluoride Acids, metal powders, flammable liquids, chlorates, nitrates, sulfur, Ammonium nitrate finely divided organics, combustibles Aniline Nitric acid, hydrogen peroxide Arsenical compounds Any reducing agent Azides Acids Ammonia, acetylene, butadiene, butane, other petroleum gases, Bromine sodium carbide, turpentine, benzene, finely divided metals Calcium oxide Water Carbon activated Calcium hypochlorite, other -

Calcium Hypochlorite
Common Name: CALCIUM HYPOCHLORITE CAS Number: 7778-54-3 DOT Number: UN 1748 (Dry or with more than 39% Chlorine) UN 2880 (Hydrated) UN 2208 (Dry with between 10% to 39% RTK Substance number: 0323 Chlorine) Date: December 1996 Revision: April 2003 ------------------------------------------------------------------------- ------------------------------------------------------------------------- HAZARD SUMMARY * Calcium Hypochlorite can affect you when breathed in. * If you think you are experiencing any work-related health * Contact can severely irritate and burn the eyes and skin. problems, see a doctor trained to recognize occupational * Breathing Calcium Hypochlorite can irritate the nose and diseases. Take this Fact Sheet with you. throat. * Breathing Calcium Hypochlorite can irritate the lungs WORKPLACE EXPOSURE LIMITS causing coughing and/or shortness of breath. Higher No occupational exposure limits have been established for exposures may cause a build-up of fluid in the lungs Calcium Hypochlorite. This does not mean that this (pulmonary edema), a medical emergency, with severe substance is not harmful. Safe work practices should always shortness of breath. be followed. IDENTIFICATION WAYS OF REDUCING EXPOSURE Calcium Hypochlorite is a white powder, granule, or pellet * Where possible, enclose operations and use local exhaust with a strong Chlorine-like odor. It is used to kill algae and ventilation at the site of chemical release. If local exhaust bacteria, in bleach and in pool chemical products. ventilation or enclosure is not used, respirators should be worn. REASON FOR CITATION * Wear protective work clothing. * Calcium Hypochlorite is on the Hazardous Substance * Wash thoroughly immediately after exposure to Calcium List because it is cited by DOT, NFPA and EPA. Hypochlorite. * Definitions are provided on page 5. -

Safe Handling and Disposal of Chemicals Used in the Illicit Manufacture of Drugs
Vienna International Centre, PO Box 500, 1400 Vienna, Austria Tel.: (+43-1) 26060-0, Fax: (+43-1) 26060-5866, www.unodc.org Guidelines for the Safe handling and disposal of chemicals used in the illicit manufacture of drugs United Nations publication USD 26 Printed in Austria ISBN 978-92-1-148266-9 Sales No. E.11.XI.14 ST/NAR/36/Rev.1 V.11-83777—September*1183777* 2011—300 Guidelines for the Safe handling and disposal of chemicals used in the illlicit manufacture of drugs UNITED NATIONS New York, 2011 Symbols of United Nations documents are composed of letters combined with figures. Mention of such symbols indicates a reference to a United Nations document. ST/NAR/36/Rev.1 UNITED NATIONS PUBLICATION Sales No. E.11.XI.14 ISBN 978-92-1-148266-9 eISBN 978-92-1-055160-1 © United Nations, September 2011. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Requests for permission to reproduce this work are welcomed and should be sent to the Secretary of the Publications Board, United Nations Headquarters, New York, N.Y. 10017, U.S.A. or also see the website of the Board: https://unp.un.org/Rights.aspx. Governments and their institutions may reproduce this work without prior authoriza- tion but are requested to mention the source and inform the United Nations of such reproduction. -
Hypochlorite Salts, As Weil As Chlorine Itself, in Aqueous Solution Produce Equilbrium Mixures of Hypochlorous Acid, Hypochlorite Ion and Chlorine
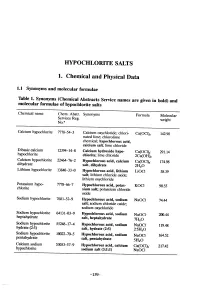
HYOCHLORITE SALTS 1. Chemical and Physical Data 1.1 Synonyms and molecular rormulae Table 1. Synonyms (Chemical Abstracts Service names are given in bold) and molecular rormulae or hyphlorite salts Chemical name Chem. Abstr. Synonyms Formula Molecular Servces Reg. weight No.u Calcium hyphlorite 7778-54-3 Calcium oxychloride; chlori- Ca(OCI)2 142.98 nated lime; chlorolime chemical; hypochlorous acid, calcium salt; lime chloride Dibasic calcium 12394-14-8 Calcium hydroxide hyp Ca(OCI)2" 291.14 hyphlorite chlonte; lime chloride 2Ca(OH)2 Calcium hyphlorite 22476-2 Hypochlorous acid, calcium Ca(OCI)2" 174.98 dihydrate salt, dihydrate 2H2O Lithium hyphlorite 1384-33-0 Hypchlorous acid, lithium LiOCI 58.39 salt; lithium chloride oxide; lithium oxychloride Potasium hyp 7778-6-7 Hypchlorous acid, potas- KOCI 90.55 chlorite sium salt; potasium chloride oxide Sodium hyphlorite 7681-52-9 Hyphlorous acid, sodium NaOCl 74.44 salt; soium chloride oxide; soium oxychloride Soium hyphlorite 64131-03-9 Hypchlorous acid, sodium heptahydrate NaOCl- 20.44 salt, heptahydrate 7H2O Sodium hyphlorite 5524-17-4 Hyphlorous acid, sodium NaOCI. 119.48 hydrate (2:5) salt, hydrate (2:5) 2-5H2O Sodium hyphlorite 10022-70-5 Hyphlorous acid, sodium NaOCI" 164.52 pentahydrate salt, pentahydrate 5H2O Calcium soium 53053-57-9 Hypchlorous acid, calcium Ca(OCI)2- 217.42 hyphlorite sodium salt (3:1:1) NaOCI -159- 160 lARe MONOGRAHS VOLUME 52 1.2 Chernical and physical properties or the pure substances From Weast (1989) unless otherwise specified Calciurn hyphlorite (a) Description: White powder or flat plates (b) Melting-point: Decomposes at 100°C (c) Density Specific gravity = 2.35 (d) Solubility: Soluble in cold water, 21.4% soluble at 25°C (Wojtowicz, 1979); insoluble in ethanol (e) Stability: Solid form decomposes exothermically when heated to 175°C, releasing oxygen (Mannsvile Chemical Products Corp., 1987). -

ECR Calcium Hypochlorite Granules EPA Reg
STORAGE AND DISPOSAL ® Read before using ECR CALCIUM Do not contaminate food or feed by storage, disposal, or cleaning of equipment. Pesticide Storage- Keep this product dry in a tightly closed container when not in use. HYPOCHLORITE Store in a cool, dry, well ventilated area away from heat or open flame. In case of decom- position, isolate container (if possible) and flood area with large amounts of water to dis- solve all materials before discarding this container. PRECAUTIONARY STATEMENTS Pesticide Disposal - Pesticide wastes may be hazardous. Improper disposal of excess HAZARDS TO HUMAN AND DOMESTIC ANIMALS GRANULES pesticide, spray mixture or rinsate is a violation of Federal Law. If these wastes cannot be disposed of use according to label instructions, contact you State Pesticide or Environ- DANGER : Highly corrosive. Causes irreversible eye damage and skin burns. Do not get in Active Ingredient: mental Control Agency, or the Hazardous Waste Representative at the nearest EPA Re- eyes, on skin, or on clothing. Wear goggles or face shield and rubber gloves when handling gional Office for guidance. this product. Wash thoroughly with soap and water after handling and before eating, drink- Calcium Hypochlorite ………………………………… 68.0% Container Disposal - Non-Refillable container. Triple rinse as follows: Empty the remain- ing, chewing gum, using tobacco, or using the toilet. Remove and wash contaminated cloth- Inert Ingredients ……………………………………… 32.0% ing contents into application equipment or a mix tank. Fill the container 1/4 full with water. ing and shoes before reuse. May be Fatal if swallowed. Irritating to nose and throat. Avoid Replace and tighten closures. -

Calcium Hypochlorite from China
UNITED STATES INTERNATIONAL TRADE COMMISSION Washington, DC Investigation Nos. 701-TA-510 and 731-TA-1245 (Final) CALCIUM HYPOCHLORITE FROM CHINA Scheduling of the final phase of countervailing duty and antidumping duty investigations. AGENCY: United States International Trade Commission. ACTION: Notice. SUMMARY: The Commission hereby gives notice of the scheduling of the final phase of antidumping and countervailing duty investigation Nos. 701-TA-510 and 731-TA-1245 (Final) under sections 705(b) and 731(b) of the Tariff Act of 1930 (19 U.S.C. '' 1671d(b) and 1673d(b)) (the Act) to determine whether an industry in the United States is materially injured or threatened with material injury, or the establishment of an industry in the United States is materially retarded, by reason of subsidized and less-than-fair-value imports from China of calcium hypochlorite, provided for in subheadings 2828.10.00, 3808.94.50, or 3808.99.95 of the Harmonized Tariff Schedule of the United States.1 For further information concerning the conduct of this phase of the investigations, hearing procedures, and rules of general application, consult the Commission=s Rules of Practice and Procedure, part 201, subparts A through E (19 CFR part 201), and part 207, subparts A and C (19 CFR part 207). EFFECTIVE DATE: Friday, July 25, 2014. FOR FURTHER INFORMATION CONTACT: Fred Ruggles (202-205-3187), Office of Investigations, U.S. International Trade Commission, 500 E Street SW, Washington, DC 20436. Hearing-impaired persons 1 For purposes of these investigations, the Department of Commerce has defined the subject merchandise as Acalcium hypochlorite, regardless of form (e.g., powder, tablet (compressed), crystalline (granular), or in liquid solution), whether or not blended with other materials, containing at least 10% available chlorine measured by actual weight. -

Busting the Myths of CHLORINE DISINFECTION
MANUFACTURING Article Busting the myths of CHLORINE DISINFECTION A successful cleanroom disinfectant needs to meet many criteria, not only in terms of its efficaciousness but also in terms of packaging, ease of use, operator acceptability, etc. Many articles have been written on how to specify and select a cleanroom disinfectant, but this is not the main focus of this article. However, a brief summary of requirements helps when comparing available chlorine chemistries. The ideal cleanroom disinfectant decontamination process is if the Taking as a given good broad spectrum disinfectant has no residue that needs to efficacy including highly resistant be removed or as a minimum is free bacterial spores, the requirements for rinsing. the ideal cleanroom disinfectant are The product will need to have in quite lengthy: a sterile option for grade A excess of a 12-month unopened shelflife and B environments1, non-flammable so and in excess of a three-month in-use it can be used over large areas with no shelflife to be practical to store and use. health and safety concerns, also fast This ideal disinfectant would then need drying with short contact times to reduce to be manufactured to the requirements the time taken for biodecontamination. of cGMP, be notified to the BPR2 and However, in an ideal world, this cannot provided in cleanroom compatible be traded for any problems with either packaging in a variety of formats so it equipment or operators and the wider was suitable for use in all areas of the environment in terms of disposal. cleanroom. It goes without saying that Another requirement shortening the this all needs to be achieved in a cost- effective formulation. -

Oxychem Sodium Hypochlorite Handbook
TABLE OF CONTENTS OxyChem Sodium Hypochlorite Handbook Introduction 2 Foreword Properties 3 This handbook outlines recommended methods for handling, storing, and using sodium hypochlorite. It also Concentration Terminology 5 includes information on the manufacture, physical properties, safety considerations and analytical methods for testing sodium Manufacturing 6 hypochlorite. Additional information and contacts can be found at www.oxychem.com Handling and Storage 9 Safety Handling 11 Unloading Tank Trucks 14 Physical Property Data 16 Methods of Analysis 18 Typical Storage Tank Installation 23 Important: The information presented herein, while not guaranteed, was prepared by technical personnel and is true and accurate to the best of our knowledge. NO WARRANTY OF MERCHANTABILITY OR OF FITNESS FOR A PARTICULAR PURPOSE, OR WARRANTY OR GUARANTY OF ANY OTHER KIND, EXPRESS OR IMPLIED, IS MADE REGARDING PERFORMANCE, SAFETY, SUITABILITY, STABILITY OR OTHERWISE. This information is not intended to be all-inclusive as to the manner and conditions of use, handling, storage, disposal and other factors that may involve other or additional legal, environmental, safety or performance considerations, and Occidental Chemical Corporation assumes no liability whatsoever for the use of or reliance upon this information. While our technical personnel will be happy to respond to questions, safe handling and use of the product remains the responsibility of the customer. No suggestions for use are intended as, and nothing herein shall be construed as, a recommendation to infringe any existing patents or to violate any Federal, State, local or foreign laws. INTRODUCTION This handbook provides information Sodium hypochlorite solutions have In 1798, Tennant of England prepared concerning sodium hypochlorite or attained widespread use in bleaching a solution of calcium hypochlorite by bleach, solutions. -

Locating and Estimating Air Emissions from Sources of Chloroform
United States Office of Air Quality EPA-450/4-84-007c Environmental Protection Planning And Standards Agency Research Triangle Park, NC 27711 March 1984 AIR EPA LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF CHLOROFORM L &E EPA- 450/4-84-007c March 1984 LOCATING & ESTIMATING AIR EMISSIONS FROM SOURCES OF CHLOROFORM U.S. ENVIRONMENTAL PROTECTION AGENCY Office of Air and Radiation Office of Air Quality Planning and Standards Research Triangle Park, North Carolina 27711 This report has been reviewed by the Office Of Air Quality Planning And Standards, U.S. Environmental Protection Agency, and has been approved for publication as received from GCA Technology. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, neither does mention of trade names or commercial products constitute endorsement or recommendation for use. ii CONTENTS Figures ...................... iv Tables ...................... v 1. Purpose of Document ............... 1 2. Overview of Document Contents .......... 3 3. Background .................... 5 Nature of Pollutant ............ 5 Overview of Production and Uses ...... 8 4. Chloroform Emission Sources ........... 11 Chloroform Production ........... 11 Fluorocarbon Production .......... 20 Pharmaceutical Manufacturing ........ 26 Ethylene Dichloride Production ....... 29 Perchloroethylene and Trichloroethylene Production . ............. 38 Chlorination of Organic Precursors in Water. 44 Miscellaneous Chloroform Emission Sources . 61 5. Source Test Procedures ............... 63 References 66 Appendix - Derivation of Emission Factors from Chloroform Production .................... A-1 References for Appendix ............... A-23 iii FIGURES Number Page 1 Chemical use tree for chloroform ............ 10 2 Basic operations that may be used in the methanol hydrochlorination/methyl chloride chlorination process 12 3 Basic operations that may be used in the methane chlorination process ................