Chemical Compatibility Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alcohols Combined 1405
ALCOHOLS COMBINED 1405 Formulas: Table 1 MW: Table 1 CAS: Table 2 RTECS: Table 2 METHOD: 1405, Issue 1 EVALUATION: PARTIAL Issue 1: 15 March 2003 OSHA : Table 2 PROPERTIES: Table 1 NIOSH: Table 2 ACGIH: Table 2 COMPOUNDS: (1) n-butyl alcohol (4) n-propyl alcohol (7) cyclohexanol (2) sec-butyl alcohol (5) allyl alcohol (8) isoamyl alcohol (3) isobutyl alcohol (6) diacetone alcohol (9) methyl isobutyl carbinol SYNONYMS: See Table 3. SAMPLING MEASUREMENT SAMPLER: SOLID SORBENT TUBE TECHNIQUE: GAS CHROMATOGRAPHY, FID (Coconut shell charcoal, 100 mg/50 mg) ANALYTE: Compounds above FLOW RATE: 0.01 to 0.2 L/min DESORPTION: 1 mL 5% 2-propanol in CS2 Compounds: (1-3 ) (4-9) VOL-MIN: 2 L 1 L INJECTION -MAX: 10 L 10 L VOLUME: 1 µL SHIPMENT: Routine TEMPERATURE -INJECTION: 220 °C SAMPLE -DETECTOR: 250 - 300 °C STABILITY: See Evaluation of Method. -COLUMN: 35 °C (7 minutes), to 60 °C at 5 °C/minute, hold 5 minutes, up to BLANKS: 2 to 10 field blanks per set 120 °C at 10 °C /minute, hold 3 minutes. CARRIER GAS: He, 4 mL/min ACCURACY COLUMN: Capillary, fused silica, 30 m x 0.32-mm RANGE STUDIED: Not studied [1, 2]. ID; 0.5 µm film polyethylene glycol, DB- wax or equivalent BIAS: Not determined CALIBRATION: Solutions of analyte in eluent (internal OVERALL standard optional) PRECISION (Ö ): Not determined rT RANGE: See EVALUATION OF METHOD. ACCURACY: Not determined ESTIMATED LOD: 1 µg each analyte per sample PRECISION: See EVALUATION OF METHOD. APPLICABILITY: This method may be used to determine two or more of the specified analytes simultaneously. -

Ethyl Glucuronide III Enzyme Immunoassay Is Intended for the • This Test Is for in Vitro Diagnostic Use Only
LZI Ethyl Glucuronide III (200) Enzyme Immunoassay – EU Only For In Vitro Diagnostic Use Only 8ºC 0530c (100/37.5 mL R1/R2 Kit) 0531c (1000/375 mL R /R Kit) 1 2 2ºC Lin-Zhi International, Inc. Intended Use Precautions and Warning The LZI Ethyl Glucuronide III Enzyme Immunoassay is intended for the • This test is for in vitro diagnostic use only. Harmful if swallowed. qualitative and semi-quantitative determination of ethyl glucuronide in human • Reagent contains sodium azide as a preservative, which may form explosive urine at the cutoff value of 200 ng/mL when calibrated against ethyl compounds in metal drain lines. When disposing such reagents or wastes, glucuronide. The assay is designed for use with a number of automated clinical always flush with a large volume of water to prevent azide build-up. See chemistry analyzers. National Institute for Occupational Safety and Health Bulletin: Explosive Azide The semi-quantitative mode is for purposes of (1) enabling laboratories to Hazards (20). determine an appropriate dilution of the specimen for confirmation by a • Do not use the reagents beyond their expiration dates. confirmatory method such as gas or liquid chromatography/mass spectrometry (GC/MS or LC/MS) or (2) permitting laboratories to establish quality control Reagent Preparation and Storage procedures. The reagents are ready-to-use. No reagent preparation is required. All assay components should be refrigerated at 2-8ºC when not in use. The assay provides only a preliminary analytical result. A more specific alternative analytical chemistry method must be used in order to obtain a Specimen Collection and Handling confirmed analytical result. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Billing Code: 3510-Ds-P Department Of
This document is scheduled to be published in the Federal Register on 01/30/2015 and available online at http://federalregister.gov/a/2015-01795, and on FDsys.gov BILLING CODE: 3510-DS-P DEPARTMENT OF COMMERCE International Trade Administration C-570-009 Calcium Hypochlorite from the People’s Republic of China: Countervailing Duty Order AGENCY: Enforcement and Compliance, International Trade Administration, Department of Commerce SUMMARY: Based on affirmative final determinations by the Department of Commerce (“Department”) and the International Trade Commission (“ITC”), the Department is issuing a countervailing duty order on calcium hypochlorite from the People’s Republic of China (“PRC”). EFFECTIVE DATE: [(Insert date of publication in the Federal Register]. FOR FURTHER INFORMATION CONTACT: Katie Marksberry, AD/CVD Operations, Office V, Enforcement and Compliance, International Trade Administration, U.S. Department of Commerce, 14th Street and Constitution Avenue, NW, Washington, DC 20230; telephone: (202) 482-7906. SUPPLEMENTARY INFORMATION: Background In accordance with section 705(d) of the Tariff Act of 1930, as amended (“the Act”), on December 15, 2014, the Department published its final determination that countervailable subsidies are being provided to producers and exporters of calcium hypochlorite from the PRC.1 On January 21, 2015, the ITC notified the Department of its final determination pursuant to 1 See Calcium Hypochlorite from the People’s Republic of China: Final Affirmative Countervailing Duty Determination; 79 FR -

THE JOURNAL of INDUSTRIAL and ENGINEERING CHEMISTRY 953 with Stirring, and the Flask Allowed to Stand
OCt., 1917 THE JOURNAL OF INDUSTRIAL AND ENGINEERING CHEMISTRY 953 with stirring, and the flask allowed to stand. The ascertaining the presence of aniline, the calcium hypo- solution, when the precipitate has settled, is filtered chlorite test is employed. The quantitative deter- through a Gooch crucible, using a bell jar arrangement, mination is carried out by precipitating the aniline into a 250 to 300 cc. Erlenmeyer flask. The crucible as tribromoaniline with bromine water. The precipi- is fitted preferably with a small circular filter paper tate is caught on a tared filter paper, dried in vacuo, (cut to size) instead of asbestos. The flask should be and weighed. washed with wash-ether (100parts absolute ether and In this connection, it occurred to the writer that it 2 parts alcoholic HC1) while filtering so as not to allow might be possible to work out, for estimating the any ferric chloride to dry on the white precipitate or aniline, a colorimetric method based on the qualita- on the crucible. The usual precautions of washing the tive test with hypochlorite. If this were possible, flask, crucible and funnel are taken to insure complete it certainly would be desirable, for not only would transfer of iron. It is not necessary, however, to trans- considerable time and effort be saved but probably fer all of the aluminum chloride precipitate to the a considerably smaller sample than IO liters would be Gooch crucible. The aluminum precipitate is removed sufficient and quantitative measurements could also with the paper from the crucible by tapping it into a be applied in cases where the amount of aniline in 25 cc. -

02/06/2019 12:05 PM Appendix 3745-21-09 Appendix A
ACTION: Final EXISTING DATE: 02/06/2019 12:05 PM Appendix 3745-21-09 Appendix A List of Organic Chemicals for which Paragraphs (DD) and (EE) of Rule 3745-21-09 of the Administrative Code are Applicable Organic Chemical Organic Chemical Acetal Benzaldehyde Acetaldehyde Benzamide Acetaldol Benzene Acetamide Benzenedisulfonic acid Acetanilide Benzenesulfonic acid Acetic acid Benzil Acetic Anhydride Benzilic acid Acetone Benzoic acid Acetone cyanohydrin Benzoin Acetonitrile Benzonitrile Acetophenone Benzophenone Acetyl chloride Benzotrichloride Acetylene Benzoyl chloride Acrolein Benzyl alcohol Acrylamide Benzylamine Acrylic acid Benzyl benzoate Acrylonitrile Benzyl chloride Adipic acid Benzyl dichloride Adiponitrile Biphenyl Alkyl naphthalenes Bisphenol A Allyl alcohol Bromobenzene Allyl chloride Bromonaphthalene Aminobenzoic acid Butadiene Aminoethylethanolamine 1-butene p-aminophenol n-butyl acetate Amyl acetates n-butyl acrylate Amyl alcohols n-butyl alcohol Amyl amine s-butyl alcohol Amyl chloride t-butyl alcohol Amyl mercaptans n-butylamine Amyl phenol s-butylamine Aniline t-butylamine Aniline hydrochloride p-tertbutyl benzoic acid Anisidine 1,3-butylene glycol Anisole n-butyraldehyde Anthranilic acid Butyric acid Anthraquinone Butyric anhydride Butyronitrile Caprolactam APPENDIX p(183930) pa(324943) d: (715700) ra(553210) print date: 02/06/2019 12:05 PM 3745-21-09, Appendix A 2 Carbon disulfide Cyclohexene Carbon tetrabromide Cyclohexylamine Carbon tetrachloride Cyclooctadiene Cellulose acetate Decanol Chloroacetic acid Diacetone alcohol -

Downloads/DL Praevention/Fachwissen/Gefahrstoffe/TOXIKOLOGI SCHE BEWERTUNGEN/Bewertungen/Toxbew072-L.Pdf
Distribution Agreement In presenting this thesis or dissertation as a partial fulfillment of the requirements for an advanced degree from Emory University, I hereby grant to Emory University and its agents the non-exclusive license to archive, make accessible, and display my thesis or dissertation in whole or in part in all forms of media, now or hereafter known, including display on the world wide web. I understand that I may select some access restrictions as part of the online submission of this thesis or dissertation. I retain all ownership rights to the copyright of the thesis or dissertation. I also retain the right to use in future works (such as articles or books) all or part of this thesis or dissertation. Signature: _____________________________ ______________ Jedidiah Samuel Snyder Date Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. Snyder Master of Public Health Global Environmental Health _________________________________________ P. Barry Ryan, Ph.D. Committee Chair _________________________________________ Eugene Demchuk, Ph.D. Committee Member _________________________________________ Paige Tolbert, Ph.D. Committee Member Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. Snyder Bachelor of Science in Engineering, B.S.E. The University of Iowa 2010 Thesis Committee Chair: P. Barry Ryan, Ph.D. An abstract of A thesis submitted to the Faculty of the Rollins School of Public Health of Emory University in partial fulfillment of the requirements for the degree of Master of Public Health in Global Environmental Health 2015 Abstract Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. -

A New Approach to Prepare Polyethylene Glycol Allyl Glycidyl Ether
E3S Web of Conferences 267, 02004 (2021) https://doi.org/10.1051/e3sconf/202126702004 ICESCE 2021 A new approach to prepare Polyethylene Glycol Allyl Glycidyl Ether Huizhen Wang1*, Ruiyang Xie1, Mingjun Chen1*, Weihao Deng1, Kaixin Zhang2, Jiaqin Liu1 1School of Science, Xihua University, Chengdu 610039, China; 2Chengdu Jingyiqiang Environmental Protection Technology Co., Ltd. Abstract. The polyethylene glycol allyl glycidyl ether (PGAGE) is an important intermediate for preparing silicone softener that can be synthesized from allyl alcohol polyoxyethylene ether and epichlorohydrin (ECH). The performance parameters including the concentration of ECH, initial boron trifluoride diethyl etherate (BFEE) as well as CaCl2 quality were investigated respectively. The optimum process parameters which can get high capping and low by-product rate are as follows: the ECH concentration is 2.0 M, the initial BFEE concentration is 1.65mM, and the CaCl2 dosage is 1.65g/L. Under these conditions, the maximal yield can be improved to 91.36%, the percent of capping rate is higher than 98.16%, the residual concentration of F- is only 0.63 mg/L. concentrated basic solution, in which the total yield was between 90%~91% by Matsuoka et al. [10] also use the 1 Introduction two-step reaction to synthesize AGE based on the reaction Polyethylene glycol allyl glycidyl ether (PGAGE) and the of allyl alcohol with ECH using BFEE as the catalyst. allyl polyoxyethylene ether (APEG), tethering with both Their results demonstrated that the yield reaches 82% alkene and epoxy groups, are widely used as fabric under the following condition: n (ECH) : n (allyl alcohol): finishing agent [1-2] , reactive diluent [3] , cross-linking (catalysis) = 1: (1~3) : (0.01~0.002). -
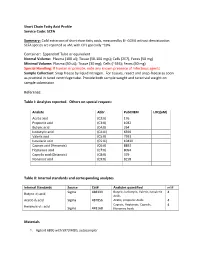
Short Chain Fatty Acid Profile Service Code: SCFA
Short Chain Fatty Acid Profile Service Code: SCFA Summary: Cold extraction of short chain fatty acids, measured by EI- GCMS without derivatization. SCFA species are reported as uM, with CV's generally ~10%. Container: Eppendorf Tube or equivalent Normal Volume: Plasma (100 ul); Tissue (50-100 mgs); Cells (2E7), Feces (50 mg) Minimal Volume: Plasma (50 uL); Tissue (30 mg); Cells (~5E6); Feces (40 mg) Special Handling: If human or primate, note any known presence of infectious agents. Sample Collection: Snap freeze by liquid nitrogen. For tissues, resect and snap-freeze as soon as practical in tared centrifuge tube. Provide both sample weight and tared vial weight on sample submission Reference: Table I: Analytes reported. Others on special request: Analyte Abbr. PubCHEM LOQ(uM) Acetic acid (C2:0) 176 Propionic acid (C3:0) 1032 Butyric acid (C4:0) 264 Isobutyric acid (C4:0i) 6590 Valeric acid (C5:0) 7991 Isovaleric acid (C5:0i) 10430 Caproic acid (Hexanoic) (C6:0) 8892 Heptanoic acid (C7:0) 8094 Caprylic acid (Octanoic) (C8:0) 379 Nonanoic acid (C9:0) 8158 Table II: Internal standards and corresponding analytes Internal Standards Source Cat# Analytes quantified mM Sigma 488399 Butyric, Isobutyric, Valeric, Isovaleric 4 Butyric-d7 acid Acids Acetic-d3 acid Sigma 487856 Acetic, propionic Acids 4 Caproic, Heptanoic, Caprylic, 4 Hexanoic-d11 acid Sigma 448168 Nonanoic Acids Materials 1. Agilent 6890 with 5973 MSD, autosampler 2. Vortexer 3. Refrigerated centrifuge, capable of 13,000g with eppendorf tube compatible rotor 4. ice bucket, ice 5. Balance 6. Prepared stock solutions of short chain fatty acid standards and isotope-labeled short chain fatty acid internal standards. -

Federal Register/Vol. 81, No. 250/Thursday, December 29, 2016
95886 Federal Register / Vol. 81, No. 250 / Thursday, December 29, 2016 / Rules and Regulations C. Regulatory Flexibility Act (RFA) I. National Technology Transfer and ENVIRONMENTAL PROTECTION Advancement Act (NTTAA) AGENCY This action is not subject to the RFA. This rulemaking does not involve The RFA applies only to rules subject to 40 CFR Part 180 notice-and-comment rulemaking technical standards. requirements under the APA, 5 U.S.C. [EPA–HQ–OPP–2016–0007 and EPA–HQ– J. Executive Order 12898: Federal OPP–2016–0008; FRL–9950–40] 553, or any other statute. This rule is not Actions To Address Environmental subject to notice-and-comment Justice in Minority Populations and Isobutyl Acetate and Isobutyric Acid; requirements because the agency has Low-Income Populations Exemption From the Requirement of a invoked the APA ‘‘good cause’’ The EPA believes that this action is Tolerance exemption under 5 U.S.C. 553(b). not subject to Executive Order 12898 (59 AGENCY: Environmental Protection FR 7629, February 16, 1994) because it D. Unfunded Mandates Reform Act Agency (EPA). (UMRA) does not establish an environmental health or safety standard. This good ACTION: Final rule. This action does not contain any cause final action simply extends the SUMMARY: This regulation establishes unfunded mandate of $100 million or date for the EPA to take action on a more as described in UMRA, 2 U.S.C. exemptions from the requirement of a petition and does not have any impact tolerance for residues of isobutyl acetate 1531–1538, and does not significantly or on human health or the environment. -

A New Coupling Process for Synthesis of Epichlorohydrin from Dichloropropanols
2nd International Conference on Machinery, Materials Engineering, Chemical Engineering and Biotechnology (MMECEB 2015) A new coupling process for synthesis of epichlorohydrin from dichloropropanols Dawei Wu, Sumin Zhou* School of Chemical Engineering, Huaiyin Institute of Technology, Huaian 223005, China [email protected] Keywords: dichloropropanol, epichlorohydrin, tubular reactor, wiped film evaporation. Abstract. As an important material used in the production of epoxy resins, epichlorohydrin is usually derived from the saponification and cyclization of dichloropropanol in a tower reacting system. In order to reduce energy consumption and wastewater discharge, a new process of tubular reactor coupled with wiped film evaporation was adopt to produce epichlorohydrin. The effects of different technological parameters on the yield of the coupling process were investigated, such as reactants' molar ratio, reaction & evaporation temperature, residence time and so on. Results indicated that the use of coupling process will significantly reduce the material consumption and shorter the reaction time. This coupling process provided a useful suggestion for the design of the industrial plants. 1. Introduction Epichlorohydyin(ECH), also known as 1-chloro-2, 3-epoxy propane, is an important chemical material, mainly used to synthesizing epoxy resin, chlorohydrins rubber, pesticide, plasticizer and so on [1]. The main method to produce ECH is high-temperature chlorination of propylene [2]. With the fast-development of biodiesel production, the use of glycerol for producing ECH [3-4] is going to mature and has become an important industrial technological process [5]. Although the difference raw materials were used, the processes also consist of two reaction steps as well. First step is the saponification and cyclization of dichloropropanol (DCP) to produce ECH. -

Synthetic Turf Scientific Advisory Panel Meeting Materials
California Environmental Protection Agency Office of Environmental Health Hazard Assessment Synthetic Turf Study Synthetic Turf Scientific Advisory Panel Meeting May 31, 2019 MEETING MATERIALS THIS PAGE LEFT BLANK INTENTIONALLY Office of Environmental Health Hazard Assessment California Environmental Protection Agency Agenda Synthetic Turf Scientific Advisory Panel Meeting May 31, 2019, 9:30 a.m. – 4:00 p.m. 1001 I Street, CalEPA Headquarters Building, Sacramento Byron Sher Auditorium The agenda for this meeting is given below. The order of items on the agenda is provided for general reference only. The order in which items are taken up by the Panel is subject to change. 1. Welcome and Opening Remarks 2. Synthetic Turf and Playground Studies Overview 4. Synthetic Turf Field Exposure Model Exposure Equations Exposure Parameters 3. Non-Targeted Chemical Analysis Volatile Organics on Synthetic Turf Fields Non-Polar Organics Constituents in Crumb Rubber Polar Organic Constituents in Crumb Rubber 5. Public Comments: For members of the public attending in-person: Comments will be limited to three minutes per commenter. For members of the public attending via the internet: Comments may be sent via email to [email protected]. Email comments will be read aloud, up to three minutes each, by staff of OEHHA during the public comment period, as time allows. 6. Further Panel Discussion and Closing Remarks 7. Wrap Up and Adjournment Agenda Synthetic Turf Advisory Panel Meeting May 31, 2019 THIS PAGE LEFT BLANK INTENTIONALLY Office of Environmental Health Hazard Assessment California Environmental Protection Agency DRAFT for Discussion at May 2019 SAP Meeting. Table of Contents Synthetic Turf and Playground Studies Overview May 2019 Update .....