Prevalence of Avian Influenza Virus Associated with Abiotic Components of Live Bird Markets in Gujranwala, Pakistan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
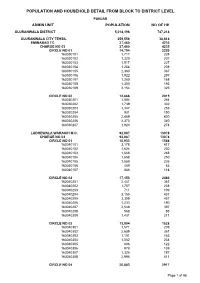
Gujranwala Blockwise
POPULATION AND HOUSEHOLD DETAIL FROM BLOCK TO DISTRICT LEVEL PUNJAB ADMIN UNIT POPULATION NO OF HH GUJRANWALA DISTRICT 5,014,196 747,214 GUJRANWALA CITY TEHSIL 259,556 38,614 EMINABAD TC 27,460 4235 CHARGE NO 03 27,460 4235 CIRCLE NO 01 14,794 2220 163030101 1,717 224 163030102 1,320 207 163030103 1,517 227 163030104 1,254 208 163030105 2,360 367 163030106 1,922 297 163030107 1,250 168 163030108 1,300 193 163030109 2,154 329 CIRCLE NO 02 12,666 2015 163030201 1,884 264 163030202 1,739 302 163030203 1,347 255 163030204 931 150 163030205 2,469 430 163030206 2,373 340 163030207 1,923 274 LUDHEWALA WARAICH M.C. 92,087 13078 CHARGE NO 04 92,087 13078 CIRCLE NO 01 10,933 1548 163040101 3,178 417 163040102 1,624 230 163040103 1,668 248 163040104 1,658 250 163040105 1,550 226 163040106 409 63 163040107 846 114 CIRCLE NO 02 17,153 2480 163040201 2,441 361 163040202 1,757 238 163040203 711 109 163040204 3,155 431 163040205 3,309 457 163040206 1,233 190 163040207 2,548 397 163040208 568 86 163040209 1,431 211 CIRCLE NO 03 13,094 1828 163040301 1,571 209 163040302 2,685 361 163040303 1,101 165 163040304 1,552 234 163040305 886 122 163040306 978 139 163040307 1,325 187 163040308 2,996 411 CIRCLE NO 04 20,883 2917 Page 1 of 98 POPULATION AND HOUSEHOLD DETAIL FROM BLOCK TO DISTRICT LEVEL PUNJAB ADMIN UNIT POPULATION NO OF HH 163040401 2,874 411 163040402 2,373 320 163040403 2,942 422 163040404 2,699 354 163040405 1,693 282 163040406 2,405 319 163040407 1,983 300 163040408 1,722 225 163040409 2,192 284 CIRCLE NO 05 18,888 2742 163040501 2,002 283 163040502 -

Details of Optic Fibre Cable (OFC) Nodes
Details of Optic Fibre Cable (OFC) Nodes S.No PROVINCE DISTRICT TEHSIL LOCATION OF OFC NODE 1 BALOCHISTAN AWARAN AWARAN AWARAN 2 BALOCHISTAN AWARAN JHAL JHAO JHAL JHAO 3 BALOCHISTAN BARKHAN BARKHAN BARKHAN 4 BALOCHISTAN BOLAN BHAG BHAG 5 BALOCHISTAN BOLAN DHADHAR DHADHAR 6 BALOCHISTAN BOLAN MACH MACH 7 BALOCHISTAN BOLAN SANNI SANNI 8 BALOCHISTAN BOLAN SANNI SHORAN 9 BALOCHISTAN CHAGHI DALBANDIN CHAGAI 10 BALOCHISTAN CHAGHI DALBANDIN DALBANDIN 11 BALOCHISTAN CHAGHI TAFTAN NOKKUNDI 12 BALOCHISTAN CHAGHI TAFTAN TAFTAN 13 BALOCHISTAN DERA BUGTI DERA BUGTI DERA BUGTI 14 BALOCHISTAN DERA BUGTI SUI SUI 15 BALOCHISTAN GWADAR GWADAR DHORE 16 BALOCHISTAN GWADAR GWADAR GWADAR 17 BALOCHISTAN GWADAR JIWANI JIWANI 18 BALOCHISTAN GWADAR ORMARA ORMARA 19 BALOCHISTAN GWADAR PASNI PASNI 20 BALOCHISTAN JAFFARABAD JHAT PAT DERA ALLAH 21 BALOCHISTAN JAFFARABAD JHAT PAT ROJHAN JAMALI 22 BALOCHISTAN JAFFARABAD USTA MOHAMMAD USTA MOHAMMAD 23 BALOCHISTAN JHAL MAGSI GANDAWA GANDAWA 24 BALOCHISTAN JHAL MAGSI JHAL MAGSI JHAL MAGSI 25 BALOCHISTAN KALAT KALAT KALAT 26 BALOCHISTAN KALAT MANGUUCHAR KHAD KOECH 27 BALOCHISTAN KALAT SURAB BAGH BANA 28 BALOCHISTAN KALAT SURAB SURAB 29 BALOCHISTAN KECH DASHT DASHT 30 BALOCHISTAN KECH KECH KALAG 31 BALOCHISTAN KECH KECH KALATUK 1 of 27 Details of Optic Fibre Cable (OFC) Nodes S.No PROVINCE DISTRICT TEHSIL LOCATION OF OFC NODE 32 BALOCHISTAN KECH KECH NASIRABAD 33 BALOCHISTAN KECH KECH NODAIZ 34 BALOCHISTAN KECH KECH PIDARAK 35 BALOCHISTAN KECH KECH TURBAT 36 BALOCHISTAN KECH TUMP BALICHAH 37 BALOCHISTAN KHARAN MASHKHEL MASHKHEL -

Understanding North Punjab in the Context of Pakistani
Jan-Mar 2011 Understanding North Punjab in t he Context of Pakistani JAN -MAR 20 11 Report Understanding North Punjab in the Context of Pakistani 0 | P a g e Conflict and Peace Studies , Volume 4, Number 1 Jan-Mar 2011 Understanding North Punjab in t he Context of Pakistani Report Understanding North Punjab in the Context of Pakistani Pak Institute for Peace Studies 1. Introduction and Background 1 A census report by the Institute for Public Policy Research in London counted that 7.53 percent of Britain’s population in 2001 was born overseas. 2 Among the top non-UK birthplaces of Britain's population, Pakistan was ranked third after Republic of Ireland and India. 3 British Pakistanis mainly hail from three parts of Pakistan and Azad Kashmir: Mirpur, which has produced more than 42 percent of their over one million population in Britain, 4 and North and Central Punjab. Many British Pakistanis also belong to Peshawar, Karachi and interior Sindh. The large-scale immigration leads to various socio-cultural, religious-political and economic transformations in both the immigrants’ native areas and the host society. To understand their behaviors and interaction with the host societies, it is important to map some prevalent socio-cultural and ideological tendencies in their areas of origin, particularly the families they belong to. This exercise becomes even more pertinent where the immigrants have maintained strong links with their native towns and also feel reluctant to be fully assimilated into their host societies for one reason or another. The primary goal of this study is to measure the key religious, ideological and political trends among Pakistani immigrants hailing from North Punjab, and some adjacent parts of Central Punjab. -

Women's Health Project
Completion Report Project Number: 27037 Loan Number: 1671 November 2007 Pakistan: Women’s Health Project CURRENCY EQUIVALENTS Currency Unit – Pakistan rupee/s (PRe/PRs) At Appraisal At Project Completion (February 1999) (December 2006) PRe1.00 = $0.0195 $0.0160 $1.00 = PRs51.30 PRs60.88 For cost comparison in this report, local currency costs were converted to US dollars at the rate prevailing at the time of each transaction. ABBREVIATIONS ADB – Asian Development Bank BCC – behavior change communication BHU – basic health unit BME – benefit monitoring and evaluation CWD – Communication and Works Department DHMT – district health management team DHQ – district headquarters DOH – Department of Health EA – executing agency EDO – executive district officer EmOC – emergency obstetric care IEC – information, education, and communication LHV – lady health visitor LHW – lady health worker MCH – maternal and child health MMR – maternal mortality rate MNCH – maternal, neonatal, and child health MOH – Ministry of Health NGO – nongovernment organization NWFP – North-West Frontier Province OPEC – Organization of Petroleum Exporting Countries PCR – project completion review PCU – project coordination unit PHN – public health nurse PSC – project steering committee RHC – rural health center RRP – report and recommendation of the President SAP – Social Action Program SDR – special drawing rights TA – technical assistance TBA – traditional birth attendant THQ – tehsil (subdistrict) headquarters TT – tetanus toxoid NOTES (i) The fiscal year (FY) of the Government of Pakistan and the provincial governments ends on 30 June. “FY” before a calendar year denotes the year in which the fiscal year ends, e.g., FY2007 ends on 30 June 2007. (ii) In this report, “$” refers to US dollars. Vice President L. -

Punjab ! ! Overview ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !
! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! - PUNJAB ! ! OVERVIEW ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! KHYBER ! ! ! ! ! PAKHTUNKHWA ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Chamba Pind ! ! ! Attock ! ! ! Hazro ! ! ! Murree ! ! ! ! Bhabra PAK ! ! ! ! ! AttockBura Hassan Abdal ! ! Wah ! Amanpura ! ! ! ! !!! ! Kotli Sattian ! A!ttock ! Bhangal ! Taxila ! ! FATA Akhori Bahlol ! ! ! ! Autrinna Mariala Bhatiot Badhana Kalan ! ! ! ! ! Rawalpindi ! ! ! ! ! !! Rawalpindi Kahuta ! Fatehjang ! ! ! Basal ! ! Morgah JAMMU & KASHMIR ! Jalwal Bango ! ! Achhral ! Band ! ! Murat ! ! Rangli Fateh Jang ! ! ATTOCK ! Adiala Gali Jagir ! Jand Abawal Bagh ! ! Bhunan Wali ! Dulehal ! ! Kallar Sayedan ! ! ! ! Bagra Arazi Chhur Mall Choha Khalsa ! ! Rawalpindi ! Jand Mandra ! !Ghalwal ! Dhok Ganganwali ! ! Malikpur ! Pari Kali ! Jhamat Dabhula ! ! Malangi ! ! Ahmadal Balawal ! ! ! ! ! ! RAWALPINDI Gujar Khan ! Saura ! Ratala ! ! Pindi Gheb ! ! Chak Beli ! !Pindi Gheb !Maghian ! Gujar Khan ! ! Malal ! ! Maira Behkhari ! ! Neela ! ! Hadawali ! ! ! Bahwaley Kallan Dora Badhal ! Dhok Afghan ! Adhi ! Makhad ! Visor ! ! ! Banth Pandori ! ! ! Dhok Abakki ! ! ! ! Shah Muhammad Wali Dewal Jamalwal Dhudial Arazi Hamid ! Bor Khui ! ! ! ! Hasola ! ! ! Multan ! ! -
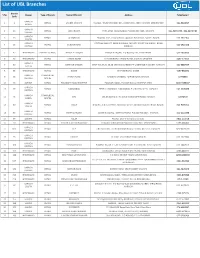
To Download UBL Branch List
List of UBL Branches Branch S No Region Type of Branch Name Of Branch Address Telephone # Code KARACHI 1 2 RETAIL LANDHI KARACHI H-G/9-D, TRUST CERAMIC IND., LANDHI IND. AREA KARACHI (EPZ) EXPORT 021-5018697 NORTH KARACHI 2 19 RETAIL JODIA BAZAR PARA LANE, JODIA BAZAR, P.O.BOX NO.4627, KARACHI. 021-32434679 , 021-32439484 CENTRAL KARACHI 3 23 RETAIL AL-HAROON Shop No. 39/1, Ground Floor, Opposite BVS School, Sadder, Karachi 021-2727106 SOUTH KARACHI CENTRAL BANK OF INDIA BUILDING, OPP CITY COURT,MA JINNAH ROAD 4 25 RETAIL BUNDER ROAD 021-2623128 CENTRAL KARACHI. 5 47 HYDERABAD AMEEN - ISLAMIC PRINCE ALLY ROAD PRINCE ALI ROAD, P.O.BOX NO.131, HYDERABAD. 022-2633606 6 46 HYDERABAD RETAIL TANDO ADAM STATION ROAD TANDO ADAM, DISTRICT SANGHAR. 0235-574313 KARACHI 7 52 RETAIL DEFENCE GARDEN SHOP NO.29,30, 35,36 DEFENCE GARDEN PH-1 DEFENCE H.SOCIETY KARACHI 021-5888434 SOUTH 8 55 HYDERABAD RETAIL BADIN STATION ROAD, BADIN. 0297-861871 KARACHI COMMERCIAL 9 65 NAPIER ROAD KASSIM CHAMBERS, NAPIER ROAD,KARACHI. 32775993 CENTRAL CENTRE 10 66 SUKKUR RETAIL FOUJDARY ROAD KHAIRPUR FOAJDARI ROAD, P.O.BOX NO.14, KHAIRPUR MIRS. 0243-9280047 KARACHI 11 69 RETAIL NAZIMABAD FIRST CHOWRANGI, NAZIMABAD, P.O.BOX NO.2135, KARACHI. 021-6608288 CENTRAL KARACHI COMMERCIAL 12 71 SITE UBL BUILDING S.I.T.E.AREA MANGHOPIR ROAD, KARACHI 32570719 NORTH CENTRE KARACHI 13 80 RETAIL VAULT Shop No. 2, Ground Floor, Nonwhite Center Abdullah Harpoon Road, Karachi. 021-9205312 SOUTH KARACHI 14 85 RETAIL MARRIOT ROAD GILANI BUILDING, MARRIOT ROAD, P.O.BOX NO.5037, KARACHI. -
Audit Report on the Accounts of City District Government Gujranwala
AUDIT REPORT ON THE ACCOUNTS OF CITY DISTRICT GOVERNMENT GUJRANWALA AUDIT YEAR 2017-18 AUDITOR GENERAL OF PAKISTAN TABLE OF CONTENTS ABBREVIATIONS & ACRONYMS ............................................................... i PREFACE ...................................................................................................... iii EXECUTIVE SUMMARY .............................................................................iv SUMMARY OF TABLES AND CHARTS.................................................. viii Table 1: Audit Work Statistics.................................................................................. viii Table 2: Audit observations regarding Financial Management .................................. viii Table 3: Outcome Statistics ...................................................................................... viii Table 4: Table of Irregularities pointed out ................................................................. ix Table 5: Cost-Benefit ................................................................................................. ix CHAPTER-1 .................................................................................................... 1 1.1 City District Government, Gujranwala ........................................................... 1 1.1.1 Introduction of Departments .......................................................................... 1 1.1.2 Comments on Budget and Accounts (Variance Analysis) ............................... 1 1.1.3 Brief Comments on the Status of Compliance -

Research Thesis on Gujranwala District
Research Thesis on Gujranwala District Research and Development Cell Gujranwala Chamber of Commerce & Industry Aiwan-e-Tijarat Road, Gujranwala, Pakistan. Phones:055-3256701-4 (4 lines), Fax:055-3254440 E-mail: [email protected], [email protected] http://www.gcci.org.pk Executive Summary The development of a nation depends upon the allocation of the resources through political institutions because the cause of justice is best served by political institutions, which unite society in a common effort to achieve an equitable distribution of resources. In rational terms, men obey state because the purpose of state is the promotion of social good or in other words greatest happiness to the greater numbers of the people. Economics touches politics at more than one point because the production and the distribution of recourses are largely influenced by government, and because the solution of many economic problems must come through political channels. Taxation. Tariff laws, government ownership of public utilities. Like railways, roads, hospitals, education, electricity, and state aid to agriculture and industry are instances where government clearly affects economic prosperity. The objective of this research is to indicate the factors that hinder the socio- political development of Gujranwala. Seeing that the city has massive potential for industrial and agricultural development even that it is facing a continuous ignorance of the ruling authorities and its socio-political development has been put in abeyance. The out come the research does need lamentation, on the injustice of the ruling authorities. There is no denying the fact if the districts like Gujranwala are paid attention the inflow of population to the big cities can be put a stop. -

Tentative Seniority List of Bs – 19 Officers (Male) Belonging to General Cadre (School Wing) Corrected Upto 31-05-2013
TENTATIVE SENIORITY LIST OF BS – 19 OFFICERS (MALE) BELONGING TO GENERAL CADRE (SCHOOL WING) CORRECTED UPTO 31-05-2013 DATE OF DATE OF DATE OF JOINING IN STY. NAME, PARENTAGE & BIRTH/ JOINING IN PLACE OF POSTING 1) BS.17 REMARKS NO. QUALIFICATION HOME TO GOVT. 2) BS.18 DISTRICT SERVICE 3) BS.19 01 02 03 04 05 06 07 1) 22-08-1989 MR. MUHAMMAD ALI PRL. GHSS, BUDHLA SANT, 01-11-1958 1. 01-05-1984 2) 24-01-1995 M.SC. B.ED MULTAN. MULTAN 3) 24-12-2001 1) 28-08-1989 MR. RASHAD ALI ZAIDI PRINCIPAL, GHSS, MANDIALA 04-07-1954 2. 10-09-1984 2) 18-09-1995 M.Sc. B.Ed. TEGA, GUJRANWALA. LAHORE 3) 12-01-2004 1) 23-08-1989 MR. AYYAZ AHMAD QURESHI SR. HM, G. ISLAMIA H/S, 03-01-1958 3. 02-05-1985 2) 24-01-1995 M.SC. M.ED MULTAN ROAD, LAHORE. LAHORE 3) 24-12-2001 1) 24-08-1989 MR. MUHAMMAD AKBAR AWAN 01-01-1959 4. SR. HM, GHS, M.GARH. 25-06-1984 2) 24-01-1995 M.SC. B.ED M.GARH 3) 24-12-2001 1) 23-08-1989 MR. NAZAR MUHAMMAD 11-9-1954 5. DEO (M-EE), BAHAWALPUR. 24-10-1985 2) 08-02-1995 M.SC.M.ED. MULTAN 3) 24-12-2001 1) 09-08-1989 MR. SHAFIQ UR REHMAN SSS, GHSS, RENALA KHURD, 20-11-1958 6. 12-12-1985 2) 24-01-1995 M.SC. M.ED OKARA. KASUR 3) 24-12-2001 1) 26-09-1989 MR. -

Urban Patterns in the Punjab Region Since Protohistoric Times
273 Reeta Grewal: Urban Patterns in Punjab Urban Patterns in the Punjab Region since Protohistoric Times Reeta Grewal Panjab University, Chandigarh _______________________________________________________________ This paper discusses the emergence, decline and revival of towns and cities in the Punjab region over the millennia. There were changes from the protohistoric Indus urbanization to the second urbanization of the early historical period. Contrary to the notion of urban decay, several new centers emerged in the region in the pre-Turkish period. The impetus to urbanization given by the Delhi Sultanate has been characterized as ‘urban revolution’. Under the Mughals, the Punjab became the third most urbanized region of the sub-continent, with a proliferation of small towns and revival of some old ones. After the decline of Mughal power there was an increase in the number of urban settlements under the emergent rulers of the late eighteenth century. The expanding state of Ranjit Singh added to the urbanscape. With the onset of colonial rule, there was a substantial increase in the number, size and functions of towns and cities, which came to dot all parts of the Punjab region. This qualitative change in the urban pattern can be termed revolutionary. While geography combined with economy had been the deciding factors in the pre-colonial times, from the colonial period onwards, polity combined with technology became crucial to the urban process. _______________________________________________________________ The Punjab region has been the site of towns and cities for nearly five thousand years. In fact, it is the area in which the first urbanization of the sub- continent emerged c.2350 BC, lasting for about 600 years upto c.1700 BC. -

PUNJAB ( X-S 4.6 M R = 23 , 50 Y 2.9 M Tahliwala Chak Miro R = 23 Or 5 Y Pakki Garhi Evel F Ater L Bowali W Narke LA 0 0
Bhagowal Dhariwal Ranganwala Chopala 11 Spur No. Chopala D oa ra Maujoke N Kassoke a la Naushahra Khoji Mahiwal Pejoki Kalan 0 Pejoki Khurd . 1 No Mari Waraichan ur Sp al jaw Sa Hakimpur Chak Mirzai 9 o. r N pu Chak Ikhtiyar r S nia Pa ak Ch Ramike Chak Paniar Lakhanwal Khurd Bohipur Moe 8 o. N r pu S r pu Bhakharwali az hb ha S Dera Pirwala Chak Rajan Arbi Mahtpur Kang Chang Saian Dera Ghulam Ali Kotha Pardhan Singh Chang Takhran Daur Shabazpur Haddoke la a N Gujar Kulla a Minuwal r ev ) a Tillu Sharqi X-Sec:ch-43fpsp2-r o yr = 237.2 m ( yr = 236.7 m, 50 D Kot Miana Water Level for 5 Nai Abadi Kotha Chishtain Nangranwala Kot Mianwala CHENAB RIVER Kothlala Kah Lala Chak Mohla Shadiwal Kot Dina Mahmudabad Tarar Dhamanwali Kartar Pur Kotli Sahuni Thatta Musa Saranke Nowshera Habibpur la Pannu Atari Na a ar Jharianwala Do Kot Sarang Dhodhuwa Jionjal Ghummanwal Jalalpur Jattan Kula Chor Kot Araian Daduwala Bhagal Jamalpur Kot Maliar NuraMandiala Rasul Pur Dera Piranwala Dera Maui Din Hussainpur Salam Garh Habibpur Dogranwala Bhakoke Sabirabad M and Mastgarh iala S pur N o. 7 Bhaggal Pindi Loharan Rasida Kotli Khokhar K Pindi Miani Kishangarh Randher More ev ) o. 6 p2-r ur N 2fps Khiwa r Sp ch-4 Hayat Garh bho Sec: Lam ( X- Kotli Khokhran Bhagowal 35 m Nasowali r = 2 Ali Akbar Abadi Sohil Kalan 50 y 9 m, Randhir 233. Giliwala yr = Lambhor or 5 vel f Kopra Khurd er Le Wat Maruf Kopra Kalan Maddoke Bawriawala Pir Kot Rambrianwala Waraichanwala Shah Jahani Gulabgarh . -

Audit Report on the Accounts of City District Government Gujranwala
AUDIT REPORT ON THE ACCOUNTS OF CITY DISTRICT GOVERNMENT GUJRANWALA AUDIT YEAR 2015-16 AUDITOR GENERAL OF PAKISTAN TABLE OF CONTENTS ABBREVIATIONS & ACRONYMS .................................................................. i PREFACE ........................................................................................................... iii EXECUTIVE SUMMARY ................................................................................ iv SUMMARY OF TABLES AND CHARTS .................................................... viii Table 1: Audit Work Statistics .................................................................................... viii Table 2: Audit observation regarding Financial Management ..................................... viii Table 3: Outcome Statistics ......................................................................................... viii Table 4: Table of Irregularities pointed out .................................................................... ix Table 5: Cost-Benefit...................................................................................................... ix CHAPTER-1 ........................................................................................................ 1 1.1 City District Government Gujranwala ............................................................... 1 1.1.1 Introduction of Departments .............................................................................. 1 1.1.2 Comments on Budget and Accounts (Variance Analysis) ................................. 1 1.1.3