Laboratory Procedures for Fish Processing and Identification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

OFFICE of RESEARCH PUBLICATIONS Please Adjust Your Settings in Acrobat to Continuous Facing to Properly View This File
YOU ARE VIEWING A .PDF FILE FROM THE OFFICE OF RESEARCH PUBLICATIONS Please adjust your settings in Acrobat to Continuous Facing to properly view this file. Thank You. CATFISH Jeff Gage Ichthyologist Larry Page with a Tiger Catfish. OME CATFISH BREATHE AIR AND SQUIGGLE ACROSS LAND.OTHERS STUN PREY WITH SSHOCKS REACHING 400 VOLTS.STILL OTHERS SUBSIST ON WOOD, LIKE TERMITES. Catfish are found on every continent except Antarctica. They range from fingernail-length miniatures to sedan- length monsters. They are among the most diverse and com- mon fishes, comprising one in four freshwater species. Despite nearly three centuries of exploration and research and the recognition of more than 2,700 species, an estimated 1,750 catfish species remain unknown to science. But not for long. Backed by a $4.7 million grant from the National Sci- ence Foundation, scientists at the University of Florida’s Florida Museum of Natural History have begun leading a five-year effort to discover and describe all catfish species. The only one of four similar projects in the NSF’s Planetary Bio- diversity Inventory program that focuses on vertebrates, the project will tap 230 scientists from around the globe, with many hauling nets and buckets into some of the world’s most remote waters. The other NSF projects focus on plants, insects and microscopic organisms called Eumycetozoa or, more commonly, slime molds. Randy Olson 18 Spring 2004 A native stalks a Suckermouth Armored Catfish in Guyana. HUNTERS BY AARON HOOVER SCIENTISTS WORLDWIDE AIM TO IDENTIFY ALL THE REMAINING SPECIES OF CATFISH, BEFORE IT’STOOLATE Practical considerations have says the goal is a comprehensive accounting before it’s too late. -

Research Funding (Total $2,552,481) $15,000 2019
CURRICULUM VITAE TENNESSEE AQUARIUM CONSERVATION INSTITUTE 175 BAYLOR SCHOOL RD CHATTANOOGA, TN 37405 RESEARCH FUNDING (TOTAL $2,552,481) $15,000 2019. Global Wildlife Conservation. Rediscovering the critically endangered Syr-Darya Shovelnose Sturgeon. $10,000 2019. Tennessee Wildlife Resources Agency. Propagation of the Common Logperch as a host for endangered mussel larvae. $8,420 2019. Tennessee Wildlife Resources Agency. Monitoring for the Laurel Dace. $4,417 2019. Tennessee Wildlife Resources Agency. Examining interactions between Laurel Dace (Chrosomus saylori) and sunfish $12,670 2019. Trout Unlimited. Southern Appalachian Brook Trout propagation for reintroduction to Shell Creek. $106,851 2019. Private Donation. Microplastic accumulation in fishes of the southeast. $1,471. 2019. AZFA-Clark Waldram Conservation Grant. Mayfly propagation for captive propagation programs. $20,000. 2019. Tennessee Valley Authority. Assessment of genetic diversity within Blotchside Logperch. $25,000. 2019. Riverview Foundation. Launching Hidden Rivers in the Southeast. $11,170. 2018. Trout Unlimited. Propagation of Southern Appalachian Brook Trout for Supplemental Reintroduction. $1,471. 2018. AZFA Clark Waldram Conservation Grant. Climate Change Impacts on Headwater Stream Vertebrates in Southeastern United States $1,000. 2018. Hamilton County Health Department. Step 1 Teaching Garden Grants for Sequoyah School Garden. $41,000. 2018. Riverview Foundation. River Teachers: Workshops for Educators. $1,000. 2018. Tennessee Valley Authority. Youth Freshwater Summit $20,000. 2017. Tennessee Valley Authority. Lake Sturgeon Propagation. $7,500 2017. Trout Unlimited. Brook Trout Propagation. $24,783. 2017. Tennessee Wildlife Resource Agency. Assessment of Percina macrocephala and Etheostoma cinereum populations within the Duck River Basin. $35,000. 2017. U.S. Fish and Wildlife Service. Status surveys for conservation status of Ashy (Etheostoma cinereum) and Redlips (Etheostoma maydeni) Darters. -

Phylogeny Classification Additional Readings Clupeomorpha and Ostariophysi
Teleostei - AccessScience from McGraw-Hill Education http://www.accessscience.com/content/teleostei/680400 (http://www.accessscience.com/) Article by: Boschung, Herbert Department of Biological Sciences, University of Alabama, Tuscaloosa, Alabama. Gardiner, Brian Linnean Society of London, Burlington House, Piccadilly, London, United Kingdom. Publication year: 2014 DOI: http://dx.doi.org/10.1036/1097-8542.680400 (http://dx.doi.org/10.1036/1097-8542.680400) Content Morphology Euteleostei Bibliography Phylogeny Classification Additional Readings Clupeomorpha and Ostariophysi The most recent group of actinopterygians (rayfin fishes), first appearing in the Upper Triassic (Fig. 1). About 26,840 species are contained within the Teleostei, accounting for more than half of all living vertebrates and over 96% of all living fishes. Teleosts comprise 517 families, of which 69 are extinct, leaving 448 extant families; of these, about 43% have no fossil record. See also: Actinopterygii (/content/actinopterygii/009100); Osteichthyes (/content/osteichthyes/478500) Fig. 1 Cladogram showing the relationships of the extant teleosts with the other extant actinopterygians. (J. S. Nelson, Fishes of the World, 4th ed., Wiley, New York, 2006) 1 of 9 10/7/2015 1:07 PM Teleostei - AccessScience from McGraw-Hill Education http://www.accessscience.com/content/teleostei/680400 Morphology Much of the evidence for teleost monophyly (evolving from a common ancestral form) and relationships comes from the caudal skeleton and concomitant acquisition of a homocercal tail (upper and lower lobes of the caudal fin are symmetrical). This type of tail primitively results from an ontogenetic fusion of centra (bodies of vertebrae) and the possession of paired bracing bones located bilaterally along the dorsal region of the caudal skeleton, derived ontogenetically from the neural arches (uroneurals) of the ural (tail) centra. -

Edna Assay Development
Environmental DNA assays available for species detection via qPCR analysis at the U.S.D.A Forest Service National Genomics Center for Wildlife and Fish Conservation (NGC). Asterisks indicate the assay was designed at the NGC. This list was last updated in June 2021 and is subject to change. Please contact [email protected] with questions. Family Species Common name Ready for use? Mustelidae Martes americana, Martes caurina American and Pacific marten* Y Castoridae Castor canadensis American beaver Y Ranidae Lithobates catesbeianus American bullfrog Y Cinclidae Cinclus mexicanus American dipper* N Anguillidae Anguilla rostrata American eel Y Soricidae Sorex palustris American water shrew* N Salmonidae Oncorhynchus clarkii ssp Any cutthroat trout* N Petromyzontidae Lampetra spp. Any Lampetra* Y Salmonidae Salmonidae Any salmonid* Y Cottidae Cottidae Any sculpin* Y Salmonidae Thymallus arcticus Arctic grayling* Y Cyrenidae Corbicula fluminea Asian clam* N Salmonidae Salmo salar Atlantic Salmon Y Lymnaeidae Radix auricularia Big-eared radix* N Cyprinidae Mylopharyngodon piceus Black carp N Ictaluridae Ameiurus melas Black Bullhead* N Catostomidae Cycleptus elongatus Blue Sucker* N Cichlidae Oreochromis aureus Blue tilapia* N Catostomidae Catostomus discobolus Bluehead sucker* N Catostomidae Catostomus virescens Bluehead sucker* Y Felidae Lynx rufus Bobcat* Y Hylidae Pseudocris maculata Boreal chorus frog N Hydrocharitaceae Egeria densa Brazilian elodea N Salmonidae Salvelinus fontinalis Brook trout* Y Colubridae Boiga irregularis Brown tree snake* -

The Evolution of the Placenta Drives a Shift in Sexual Selection in Livebearing Fish
LETTER doi:10.1038/nature13451 The evolution of the placenta drives a shift in sexual selection in livebearing fish B. J. A. Pollux1,2, R. W. Meredith1,3, M. S. Springer1, T. Garland1 & D. N. Reznick1 The evolution of the placenta from a non-placental ancestor causes a species produce large, ‘costly’ (that is, fully provisioned) eggs5,6, gaining shift of maternal investment from pre- to post-fertilization, creating most reproductive benefits by carefully selecting suitable mates based a venue for parent–offspring conflicts during pregnancy1–4. Theory on phenotype or behaviour2. These females, however, run the risk of mat- predicts that the rise of these conflicts should drive a shift from a ing with genetically inferior (for example, closely related or dishonestly reliance on pre-copulatory female mate choice to polyandry in conjunc- signalling) males, because genetically incompatible males are generally tion with post-zygotic mechanisms of sexual selection2. This hypoth- not discernable at the phenotypic level10. Placental females may reduce esis has not yet been empirically tested. Here we apply comparative these risks by producing tiny, inexpensive eggs and creating large mixed- methods to test a key prediction of this hypothesis, which is that the paternity litters by mating with multiple males. They may then rely on evolution of placentation is associated with reduced pre-copulatory the expression of the paternal genomes to induce differential patterns of female mate choice. We exploit a unique quality of the livebearing fish post-zygotic maternal investment among the embryos and, in extreme family Poeciliidae: placentas have repeatedly evolved or been lost, cases, divert resources from genetically defective (incompatible) to viable creating diversity among closely related lineages in the presence or embryos1–4,6,11. -

Peoria Riverfront Development, Illinois (Ecosystem Restoration)
PEORIA RIVERFRONT DEVELOPMENT, ILLINOIS (ECOSYSTEM RESTORATION) FEASIBILITY STUDY WITH INTEGRATED ENVIRONMENTAL ASSESSMENT MAIN REPORT MARCH 2003 DEPARTMENT OF THE ARMY ROCK ISLAND DISTRICT, CORPS OF ENGINEERS CLOCK TOWER BUILDING - P.O. BOX 2004 ROCK ISLAND, ILLINOIS 61204-2004 REPLY TO ATTENTION OF http://www.mvr.usace.army.mil CEMVR-PM-M PEORIA RIVERFRONT DEVELOPMENT, ILLINOIS (ECOSYSTEM RESTORATION) FEASIBILITY STUDY WITH INTEGRATED ENVIRONMENTAL ASSESSMENT MAIN REPORT MARCH 2003 Executive Summary he Peoria Riverfront Development (Ecosystem Restoration) Project area includes Lower Peoria Lake. The area lies within Peoria and Tazewell Counties, Illinois, and includes TIllinois River Miles 162-167. The project is related to the Peoria Riverfront Development Project, a public and private cooperative effort that also includes revitalization of the City’s downtown area. Development includes a visitor’s center, city park, residential redevelopment, community center, riverboat landing, sports complex, entertainment centers, and retail development. The region has begun to reclaim its abandoned industrial riverfront, with the understanding that a healthy, attractive, and sustainable environment must be present. The Illinois River is a symbol of the region’s economic, social, and cultural history, as well as its future. Therefore, ecosystem restoration in Peoria Lake is a vital component to an overall effort and vision to develop the Peoria Riverfront in an ecologically, economically, and socially sustainable manner. This Ecosystem Restoration Feasibility Study was conducted by the U.S. Army Corps of Engineers and the Illinois Department of Natural Resources (Non-Federal Sponsor) to investigate the Federal and State interest in ecosystem restoration within Peoria Lake. In support of this resource vision, several regulatory efforts on the part of the cities and counties to address the sedimentation issue affecting the Illinois River have been adopted. -

Ecology and Conservation of Mudminnow Species Worldwide
FEATURE Ecology and Conservation of Mudminnow Species Worldwide Lauren M. Kuehne Ecología y conservación a nivel mundial School of Aquatic and Fishery Sciences, University of Washington, Seattle, WA 98195 de los lucios RESUMEN: en este trabajo, se revisa y resume la ecología Julian D. Olden y estado de conservación del grupo de peces comúnmente School of Aquatic and Fishery Sciences, University of Washington, Box conocido como “lucios” (anteriormente conocidos como 355020, Seattle, WA 98195, and Australian Rivers Institute, Griffith Uni- la familia Umbridae, pero recientemente reclasificados en versity, QLD, 4111, Australia. E-mail: [email protected] la Esocidae) los cuales se constituyen de sólo cinco espe- cies distribuidas en tres continentes. Estos peces de cuerpo ABSTRACT: We review and summarize the ecology and con- pequeño —que viven en hábitats de agua dulce y presentan servation status of the group of fishes commonly known as movilidad limitada— suelen presentar poblaciones aisla- “mudminnows” (formerly known as the family Umbridae but das a lo largo de distintos paisajes y son sujetos a las típi- recently reclassified as Esocidae), consisting of only five species cas amenazas que enfrentan las especies endémicas que distributed on three continents. These small-bodied fish—resid- se encuentran en contacto directo con los impactos antro- ing in freshwater habitats and exhibiting limited mobility—often pogénicos como la contaminación, alteración de hábitat occur in isolated populations across landscapes and are subject e introducción de especies no nativas. Aquí se resume el to conservation threats common to highly endemic species in conocimiento actual acerca de la distribución, relaciones close contact with anthropogenic impacts, such as pollution, filogenéticas, ecología y estado de conservación de cada habitat alteration, and nonnative species introductions. -

Darter Reproductive Seasons Author(S): Clark Hubbs Reviewed Work(S): Source: Copeia, Vol
Darter Reproductive Seasons Author(s): Clark Hubbs Reviewed work(s): Source: Copeia, Vol. 1985, No. 1 (Feb. 11, 1985), pp. 56-68 Published by: American Society of Ichthyologists and Herpetologists (ASIH) Stable URL: http://www.jstor.org/stable/1444790 . Accessed: 10/01/2012 14:26 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. American Society of Ichthyologists and Herpetologists (ASIH) is collaborating with JSTOR to digitize, preserve and extend access to Copeia. http://www.jstor.org 56 COPEIA, 1985, NO. 1 changes in kinosternid turtles. J. Herpetol. 6:183- . 1938. Seasonal changes in the testes of the 189. musk turtle Sternotherusodoratus L. J. Morphol. 63: MCPHERSON, R. J., AND K. R. MARION. 1981. Sea- 301-317. sonal testicular cycle of the stinkpot turtle (Ster- SAINTGIRONS, H. 1982. Reproductive cycles of male notherus odoratus) in central Alabama. Herpetolog- snakes and their relationships with climate and fe- ica 37:33-40. male reproductive cycles. Herpetologica 38:5-16. MITCHELL, J. C. 1982. Population ecology and de- SPEAT, R. H. 1973. Seasonal variation in the tubular mography of the freshwater turtles Chrysemyspicta and interstitial areas of the testes in Sternothaerus and Sternotherusodoratus. -

The Life History of the Slough Darter, Etheostoma Gracile (Pisces, Percidae)
• 7iz THE LIFE HISTORY OF THE SLOUGH DARTER, ETHEOSTOMA GRACILE (PISCES, PERCIDAE) Marvin E. Braasch Philip W. Smith ILLINOIS NATURAL HISTORY SURVEY Biological Notes No. 58 Urbana, Illinois • June, 1967 State of Illinois Department of Registration and Education NATURAL HISTORY SURVEY DIVISION THE LIFE HISTORY OF THE SLOUGH DARTER, ETHEOSTOMA GRACILE (PISCES, PERCIDAE) Marvin E. Braasch and Philip W. Smith SEVERAL STUDIES HAVE BEEN PUBLISHED pore, but in the female the genital pore is distinctly on reproductive habits of darters (for a summary, see larger than the anal pore. Winn 1958) . However, a detailed life-history study The small young of the species can be readily dis- is not available for any of the eight species and sub- tinguished from juveniles of other darters occurring species of the subgenus Hololepis. The subgenus is an with them by the distinctly reddish eye, three small ecologically distinctive group of which all members typi- caudal spots, and pronounced upward flexure of the cally inhabit swamps, sloughs, and low-gradient streams groove for the lateral line. in the Coastal Plain and Mississippi River valley. The species was described by Girard (1859:103) as A surprising amount of ecological information has, Boleosoma gracile (type-locality Rio Seco, Fort Inge, nevertheless, been assembled by Hubbs & Cannon (1935) Uvalde County, Texas) and, until Bailey (1951) re- and especially by Collette (1962) through remarkably duced many nominal genera to subgeneric rank, was thorough reviews of others' published observations and variously placed in the genera Boleosoma, Boleichthys, through inferences drawn from morphology. This paper Poecilichthys, and Hololepis. Poecilichthys butlerianus on the slough darter, Etheostoma gracile (Girard) , the Hay, 1882 (type-locality Big Black River, Yazoo County, westernmost member of the subgenus, substantiates many Mississippi) (1882:61) and Poecilichthys palustris Gil- of Collette's (1962) inferences and supplies some miss- bert, 1884 (type-locality Switz City swamp, Greene ing details. -
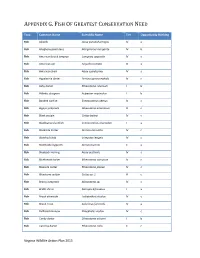
Fish of Greatest Conservation Need
APPENDIX G. FISH OF GREATEST CONSERVATION NEED Taxa Common Name Scientific Name Tier Opportunity Ranking Fish Alewife Alosa pseudoharengus IV a Fish Allegheny pearl dace Margariscus margarita IV b Fish American brook lamprey Lampetra appendix IV c Fish American eel Anguilla rostrata III a Fish American shad Alosa sapidissima IV a Fish Appalachia darter Percina gymnocephala IV c Fish Ashy darter Etheostoma cinereum I b Fish Atlantic sturgeon Acipenser oxyrinchus I b Fish Banded sunfish Enneacanthus obesus IV c Fish Bigeye jumprock Moxostoma ariommum III c Fish Black sculpin Cottus baileyi IV c Fish Blackbanded sunfish Enneacanthus chaetodon I a Fish Blackside darter Percina maculata IV c Fish Blotched chub Erimystax insignis IV c Fish Blotchside logperch Percina burtoni II a Fish Blueback Herring Alosa aestivalis IV a Fish Bluebreast darter Etheostoma camurum IV c Fish Blueside darter Etheostoma jessiae IV c Fish Bluestone sculpin Cottus sp. 1 III c Fish Brassy Jumprock Moxostoma sp. IV c Fish Bridle shiner Notropis bifrenatus I a Fish Brook silverside Labidesthes sicculus IV c Fish Brook Trout Salvelinus fontinalis IV a Fish Bullhead minnow Pimephales vigilax IV c Fish Candy darter Etheostoma osburni I b Fish Carolina darter Etheostoma collis II c Virginia Wildlife Action Plan 2015 APPENDIX G. FISH OF GREATEST CONSERVATION NEED Fish Carolina fantail darter Etheostoma brevispinum IV c Fish Channel darter Percina copelandi III c Fish Clinch dace Chrosomus sp. cf. saylori I a Fish Clinch sculpin Cottus sp. 4 III c Fish Dusky darter Percina sciera IV c Fish Duskytail darter Etheostoma percnurum I a Fish Emerald shiner Notropis atherinoides IV c Fish Fatlips minnow Phenacobius crassilabrum II c Fish Freshwater drum Aplodinotus grunniens III c Fish Golden Darter Etheostoma denoncourti II b Fish Greenfin darter Etheostoma chlorobranchium I b Fish Highback chub Hybopsis hypsinotus IV c Fish Highfin Shiner Notropis altipinnis IV c Fish Holston sculpin Cottus sp. -

Ambloplites Constellatus, a New Species of Rock Bass from the Ozark Upland of Arkansas and Missouri with a Review of Western Rock Bass Populations
Ambloplites constellatus, a New Species of Rock Bass from the Ozark Upland of Arkansas and Missouri with a Review of Western Rock Bass Populations ROBERT C. CASHNER and ROYAL D. SUTTKUS V Reprinted from THE AMERICAN MIDLAND NATURALIST Vol. 98, No. 1, July, 1977, pp. 147-161 University of Notre Dame Press Notre Dame, Indiana Ambloplites constellatus, a New Species of Rock Bass from the Ozark Upland of Arkansas and Missouri with a Review of Western Rock Bass Populations' ROBERT C. CASHNER Department of Biological Sciences, University of New Orleans, New Orleans, Louisiana 70122 and ROYAL D. SUTTKUS Tulane University, Museum of Natural History, Belle Chasse, Louisiana 70037 ABSTRACT: A new species of rock bass, Ambloplites constellatus, is described from the upland section of the White River in Arkansas and Missouri. It is compared with the closely related northern rock bass (A. rupestris) from Missouri and Meramec river populations, the southern rock bass (A. ariommus) from the Ouachita and Little river drainages, and with other western rock bass populations of undetermined status. Ambloplites constellatus is distinguished from its congeners by its freckled color pattern and slender body form. Ambloplites constellatus occurs throughout the upper White River. There are two records of the species from the Osage River drainage in Missouri. INTRODUCTION In his study of Missouri fishes, Pflieger (1971) noted that rock bass from the upper White River system differed strikingly in color pattern from other Missouri populations. Based on our examination of mate- rial from throughout the Ozark Upland province, as well as other western rock bass populations, we describe the upper White River population as a new species, Ambloplites constellatus, the Ozark rock bass. -

Information on the NCWRC's Scientific Council of Fishes Rare
A Summary of the 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes in North Carolina Submitted by Bryn H. Tracy North Carolina Division of Water Resources North Carolina Department of Environment and Natural Resources Raleigh, NC On behalf of the NCWRC’s Scientific Council of Fishes November 01, 2014 Bigeye Jumprock, Scartomyzon (Moxostoma) ariommum, State Threatened Photograph by Noel Burkhead and Robert Jenkins, courtesy of the Virginia Division of Game and Inland Fisheries and the Southeastern Fishes Council (http://www.sefishescouncil.org/). Table of Contents Page Introduction......................................................................................................................................... 3 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes In North Carolina ........... 4 Summaries from the 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes in North Carolina .......................................................................................................................... 12 Recent Activities of NCWRC’s Scientific Council of Fishes .................................................. 13 North Carolina’s Imperiled Fish Fauna, Part I, Ohio Lamprey .............................................. 14 North Carolina’s Imperiled Fish Fauna, Part II, “Atlantic” Highfin Carpsucker ...................... 17 North Carolina’s Imperiled Fish Fauna, Part III, Tennessee Darter ...................................... 20 North Carolina’s Imperiled Fish Fauna, Part