Peripheral Giant Cell Granuloma in a Dog
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prevalence of Oral Lesions in Complete Denture Wearers- an Original Research
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 20, Issue 1 Ser.3 (January. 2021), PP 29-33 www.iosrjournals.org Prevalence of oral lesions in complete denture wearers- An original research Prenika Sharma1*, Reecha Gupta2 1- MDS, Oral medicine and radiology 2- Professor and HOD Department of Prosthodontics, Indira Gandhi Govt. Dental College, Jammu (J&K) Abstract: Background: Complete denture patients are often associated with the various denture-related oral mucosallesions. The purpose of this study is to evaluate the prevalence ofdenture-related oral mucosal lesions in complete denture patients. Materials and Methods: The study was consisted of 225 patientshaving various denture-induced oral mucosal lesions from theoutpatient department of the department out of the 395 completedenture patients examined. Data related to gender, age, length ofdenture use, hygiene care were obtained. All the data were tabulated and analyzed. Results: In 225 complete denture patients. Denture stomatitis (60.23%) was the most commonlesion present, followed by Epulis fissuratum and angularcheilitis. The denture-induced oral mucosal lesions werefound more common in age >40 years (59.78%) and in female(52.70%) complete denture wearer patients. Conclusion: The present studies showed that oral lesions associated with wearing denture are prevalent and create health problems that impact the quality of life of dental patients. Key Words: Complete denture, denture stomatitis, Epulis fissuratum, oralmucosal lesions. --------------------------------------------------------------------------------------------------------------------------------------- Date of Submission: 26-12-2020 Date of Acceptance: 07-01-2021 --------------------------------------------------------------------------------------------------------------------------------------- I. Introduction Edentulism may be the last sequel of periodontal diseases and dental caries. In case of older adults, edentulism is essential as a correlate of self-esteem and quality of life. -

Application of Lasers in Treatment of Oral Premalignant Lesions
Symbiosis www.symbiosisonline.org www.symbiosisonlinepublishing.com Review article Journal of Dentistry, Oral Disorders & Therapy Open Access Application of Lasers in Treatment of Oral Premalignant Lesions Amaninder Singh*1, Akanksha Zutshi2, Preetkanwal Singh Ahluwalia3, Vikas Sharma4 and Vandana Razdan5 1,4oral and maxillofacial surgery, reader, National Dental College and Hospital, Dera Bassi, Punjab 2oral and maxillofacial surgery, senior lecturer, National Dental College and Hospital, Dera Bassi, Punjab 3oral and maxillofacial surgery, professor, National Dental College and Hospital, Dera Bassi, Punjab 5Pharmacology, professor, Govt. Medical College and Hospital, Jammu Received: April 03, 2018; Accepted: June 04, 2018; Published: June 11, 2018 *Corresponding author: Amaninder Singh, House No- 620, Phase- 6, mohali, 160055, E-mail address: [email protected] Abstract radiation. Laser systems and their application in dentistry and especially the basis of energy of the beam and wavelength of the emitted oral surgery are rapidly improving today. Lasers are being used as a niche tool as direct replacement for conventional approaches ClassificationGas lasers of lasers [6] CO advantages of lasers are incision of tissues, coagulation during Argon like scalpel, blades, electro surgery, dental hand piece. The specific Liquid Dyes2 canoperation be used and for treatmentpostoperative of conditions benefits likesuch lowas premalignant postoperative lesions, pain, better wound healing. For soft tissue oral surgical procedures lasers Solid -

Supernumerary Teeth in Primary Dentition Associated to Palatal Polyps
Revista Odontológica Mexicana Facultad de Odontología Vol. 17, No. 3 July-September 2013 pp 168-172 CASE REPORT Supernumerary teeth in primary dentition associated to palatal polyps. Case report Dientes supernumerarios en dentición primaria asociados a pólipos palatinos. Reporte de caso Thalia Sánchez Muñoz Ledo,* Alejandro Hinojosa Aguirre,§ Germán Portillo Guerrero,II Fernando Tenorio Rocha¶ ABSTRACT RESUMEN Polyps are rare in children. The present article reports the clinical Los pólipos son poco frecuentes en niños. En este artículo se pre- case of a 14 month old male patient brought for treatment to the senta un caso clínico de un niño de un año dos meses que acude Pedodontics Clinic of the Graduate School, National School of a la Clínica de Odontopediatría de la DEPeI UNAM con dos póli- Dentistry National University of Mexico. He presented two palatal pos fibroepiteliales palatinos ubicados a ambos lados de la papila fibro-epithelial polyps, located at both sides of the incisive papilla. incisiva, 10 días posteriores a la excisión quirúrgica se observó la 10 days after surgical excision, a supernumerary tooth erupted in erupción de un diente supernumerario en el paladar, y 25 días des- the palate. 25 days later, eruption of a second supernumerary tooth pués se observó la erupción de un segundo diente supernumerario. was observed. Both teeth were extracted. Histological diagnosis Ambos dientes fueron extraídos. El diagnóstico histológico de las of palatal lesions was giant fibroblast fibroma. Nevertheless, no lesiones en el paladar fue: fibroma de fibroblastos gigantes; sin -em histological evidence was found to show possible relationship bargo, no se encontró evidencia histológica que mostrara alguna between presence of palatal polyps and supernumerary teeth. -

The Peripheral Giant Cell Granuloma in Edentulous Patients: Report of Three Unique Cases
Published online: 2019-09-30 The Peripheral Giant Cell Granuloma in Edentulous Patients: Report of Three Unique Cases Osman A. Etoza Ahmet Emin Demirbasa Mehmet Bulbulb Ebru Akayc ABSTRACT The peripheral giant cell granuloma (PGCG) is a rare reactive exophytic lesion taking place on the gingiva and alveolar ridge usually as a result of local irritating factors such as trauma, tooth extrac- tion, badly finished fillings, unstable dental prosthesis, plaque, calculus, chronic infections, and im- pacted food. This article presents 3 cases of PGCG that presented at the same location of the edentu- lous mandible of patients that using complete denture for over ten years. (Eur J Dent 2010;4:329-333) Key words: Peripheral giant cell granuloma; Chronic irritation; Edentulous patients; Complete denture. INTRODUCTION Giant cell granuloma lesions (peripheral and teoclastoma, or giant-cell hyperplasia. Etiologic central) are benign, non-odontogenic, moderately factors are not known, although it is thought that rare tumors of the oral cavity. They develop pe- it may be due to an irritant or aggressive factor ripherally (within gingiva) or centrally (in bone).1 such as trauma, tooth extraction, badly finished The peripheral giant cell granuloma (PGCG) is a fillings, unstable dental prosthesis, plaque, calcu- rare reactive exophytic lesion taking place on the lus, chronic infections, or impacted food.2,3 Clini- gingiva and alveolar ridge, also known as a giant- cal appearance of PGCGs can present as polyploi- cell epulis, giant-cell reparative granuloma, os- dy or nodular lesions, primarily bluish red with a smooth shiny or mamillated surface, stalky or 2,4,5 a Erciyes University, Faculty of Dentistry, Department of sessile base, small and well demarcated. -

Pigmented Villonodular Synovitis of the Temporomandibular Joint: a Case Report and the Literature Review
1314 Cai et al. Case Report TMJ Disorders J. Cai1, Z. Cai1, Y. Gao2 1Department of Oral and Maxillofacial Pigmented villonodular synovitis 2 Surgery, Beijing, China; Department of Oral Pathology, Peking University School & of the temporomandibular joint: Hospital of Stomatology, Beijing, China a case report and the literature review J. Cai, Z. Cai, Y. Gao: Pigmented villonodular synovitis of the temporomandibular joint: a case report and the literature review. Int. J. Oral Maxillofac. Surg. 2011; 40: 1314–1322. # 2011 International Association of Oral and Maxillofacial Surgeons. Published by Elsevier Ltd. All rights reserved. Abstract. Pigmented villonodular synovitis (PVNS) is an uncommon benign proliferative disorder of synovium that may involve joints, tendon sheaths, and bursae. It most often affects the knees, and less frequently involves other joints. It presents in the temporomandibular joints (TMJs) extremely rarely. The authors report an elderly female patient with PVNS of the TMJ with skull base extension, who had traumatic history in the same site. It was diagnosed through core-needle Keywords: pigmented villonodular synovitis (PVNS); synovitis; temporomandibular joint biopsy, which was not documented in the literature. Radical excision and follow-up (TMJ). for 7–8 years was recommended because of the reported malignant transformation and high recurrence rate. This case and previously reported cases in the literature are Accepted for publication 2 March 2011 reviewed and discussed. Available online 6 April 2011 The term pigmented villonodular synovi- were reported in detail (Table 1). The visits and mouth-opening for a long time tis (PVNS) was introduced by JAFFE et al. authors present an additional case of during the operation. -

Multiple Large Peripheral Giant Cell Granuloma: a Case Report
CASE REPORT BALIKESİR SAĞLIK BİLİMLERİ DERGİSİ / BALIKESIR HEALTH SCIENCES JOURNAL MULTIPLE LARGE PERIPHERAL GIANT CELL GRANULOMA: A CASE REPORT BÜYÜK BOYUTLARDA MULTİPL PERİFERAL DEV HÜCRELI GRANÜLOM: VAKA RAPORU Mustafa Gümüşok1 Murat Özle2 Begüm Okur2 Anıl Seçkin2 Farid Museyibov3 Özlem Üçok1 Sedat Çetiner2 1Gazi Üniversitesi Diş Hekimliği Fakültesi, ÖZET Ağız, Diş Ve Çene Radyolojisi Anabilim Dalı, Ankara Periferal dev hücreli granülom (PDHG) oral bölgenin sık izlenen dev hücreli bir lezyonudur. 2Gazi Üniversitesi Diş Hekimliği Fakültesi, PDHG’ler gerçek bir neoplazmı temsil etmezler. Etyolojileri çok açık olmayan bu lezyonların, Ağız, Diş Ve Çene Cerrahisi Anabilim Dalı, travma veya lokal irritasyonlara bağlı gelişen reaktif bir lezyon olduğu düşünülmektedir. Ankara PDHG’lere kadınlarda erkeklere oranla daha sık, mandibulada ise maksilladan daha fazla 3 Gazi Üniversitesi Diş Hekimliği Fakültesi, rastlanılır. Gingiva veya dişsiz alveolar kret üzerinde kırmızı, kırmızı-mavi nodüler kitle şeklinde Oral Patoloji Anabilim Dalı, Ankara görülürler. Bu olgu raporunda, 48 yaşında erkek hastada görülen, maksilla sol santral kesici - sağ kanin kesici dişler ve mandibula sağ santral kesici-sol kanin kesici dişler bölgesinde lokalize, Yazışma Adresi: yüzde asimetriye neden olan büyük boyutlu PDHG’ler sunulmuştur. Multipl PDHG vakasının Mustafa Gümüşok radyolojik, klinik, histopatolojik bulguları ile birlikte teşhis, tedavi ve 6 aylık takibi rapor Gazi Üniversitesi Diş Hekimliği Fakültesi Ağız edilmiştir. Diş Ve Çene Radyolojisi Asti Karşısı Emek Ankara Ankara – Türkiye Anahtar Kelimeler: Periferal dev hücreli granülom, mandibula, maksilla, multipl lezyon SUMMARY E posta: [email protected] Peripheral giant cell granuloma (PGCG) is the most common oral giant cell lesion. PGCG Kabul Tarihi: 25 Şubat 2015 presumably does not represent a true neoplasm. PGCG is believed to be stimulated by local irritation or trauma besides the causing of PGCG isn’t known exactly. -
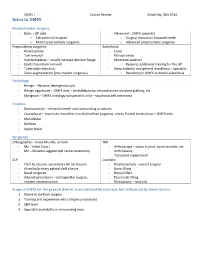
Intro to OMFS
OMFS I Course Review Enoch Ng, DDS 2014 Intro to OMFS Dentoalveolar surgery - Basic – GP able - Advanced – OMFS specialist o Extraction of erupted o Surgical extraction impacted teeth o Minor preprosthetic surgeries o Advanced preprosthetic surgeries Preprosthetic surgeries Anesthesia - Alveoloplasty - Local - Tori removal - Nitrous oxide - Vestibuloplasty – usually increase denture flange - Moderate sedation - Epulis fissuratum removal o Requires additional training for the GP - Tuberosity reduction - Deep sedation and general anesthesia – specialist - Sinus augmentation (pre-implant surgeries) o Residency in OMFS or dental anesthesia Pathology - Benign – fibroma, dentigerous cyst - Benign aggressive – OMFS only – ameloblastoma, reconstruction via bone grafting, etc - Malignant – OMFS oncology subspecialist only – squamous cell carcinoma Trauma - Dentoalveolar – related to teeth and surrounding structures - Craniofacial – trauma to mandible, maxilla/midface (zygoma), orbits, frontal bones/sinus – OMFS only - Mandibular - Midface - Upper facial Surgeries Orthognathic – move Mx, Mn, or both TMJ - Mx – lefort Class I - Arthroscopy – scope in joint, inject steroids, etc - Mn – bilateral saggital split ramus osteotomy - Arthroplasty - Total joint replacement CLP Cosmetic - Cleft lip closure, secondary cleft lip closure - Blepharoplasty – eye lid surgery - Alveolar/primary palatal cleft closure - Brow lifting - Nasal surgeries - Botox/fillers - Adjunct procedures – orthognathic surgery, - Face/neck lifting implant reconstruction - Rhinoplasty -

Peripheral Giant Cell Granuloma-A Rare Oral Entity
Case Report Adv Dent & Oral Health Volume 6 Issue 4 - December 2017 Copyright © All rights are reserved by Karthikeyan Ramalingam DOI: 10.19080/ADOH.2017.06.555694 Peripheral Giant Cell Granuloma-A Rare Oral Entity Karthikeyan Ramalingam*, Sandeep Goyal and Sathya Sethuraman Department of Oral Pathology and Microbiology, Surendera Dental College and Research Institute, Rajasthan, India Submission: October 26, 2016; Published: December 04, 2017 *Corresponding author: Karthikeyan Ramalingam, Department of Oral Pathology and Microbiology, Surendera Dental College and Research Institute, Rajasthan, India, Email: Abstract Peripheral Giant Cell granuloma (PGCG) is one of the hyperplastic lesions of the oral cavity. It could arise from the periosteum or the periodontal membrane subsequent to chronic trauma or local irritation. It accounts for less than 10% of all hyperplastic gingival lesions, rarely exceeds 2cm in size and predominantly noted in females. We report a rare case of a large PGCG involving the right mandibular anterior gingiva stroma along with extravasated RBCs in histopathology. The lesion was surgically excised and the patient is remaining disease free on follow-up. in a 26-year-old male patient of Indian origin. It presented as a pinkish-red nodule which showed multinucleated giant cells in fibrous cellular Keywords: Peripheral giant cell granuloma; Mandibular gingival; Males; Anterior region Key Messages: We report the case of peripheral giant cell granuloma in the mandibular anterior gingiva in a male patient. This rare entity should be kept in mind on encountering such hyperplastic lesions in the oral cavity. Introduction multinucleated giant cells along with hemosiderin deposits and Peripheral giant cell granuloma (PGCG) is the infrequent, exophytic oral lesion that commonly contains giant cells. -

Periodontal Health and Gingival Diseases
Received: 9 December 2017 Revised: 11 March 2018 Accepted: 12 March 2018 DOI: 10.1002/JPER.17-0719 2017 WORLD WORKSHOP Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions Iain L.C. Chapple1 Brian L. Mealey2 Thomas E. Van Dyke3 P. Mark Bartold4 Henrik Dommisch5 Peter Eickholz6 Maria L. Geisinger7 Robert J. Genco8 Michael Glogauer9 Moshe Goldstein10 Terrence J. Griffin11 Palle Holmstrup12 Georgia K. Johnson13 Yvonne Kapila14 Niklaus P. Lang15 Joerg Meyle16 Shinya Murakami17 Jacqueline Plemons18 Giuseppe A. Romito19 Lior Shapira10 Dimitris N. Tatakis20 Wim Teughels21 Leonardo Trombelli22 Clemens Walter23 Gernot Wimmer24 Pinelopi Xenoudi25 Hiromasa Yoshie26 1Periodontal Research Group, Institute of Clinical Sciences, College of Medical & Dental Sciences, University of Birmingham, UK 2University of Texas Health Science Center at San Antonio, USA 3The Forsyth Institute, Cambridge, MA, USA 4School of Dentistry, University of Adelaide, Australia 5Department of Periodontology and Synoptic Dentistry, Charité - Universitätsmedizin Berlin, Germany 6Department of Periodontology, Center for Oral Medicine, Johann Wolfgang Goethe-University Frankfurt, Germany 7Department of Periodontology, University of Alabama at Birmingham, USA 8Department of Oral Biology, SUNY at Buffalo, NY, USA 9Faculty of Dentistry, University of Toronto, Canada 10Department of Periodontology, Faculty of -

Pathology of Oral Cavity
Developmental disorders Facial cleft – disorder in the migration of neural crest cells failure of the fusion process and failure of inwards growth of the mesoderm genetics play a part in the development of facial clefts Lateral cleft –the commonest -cheiloschisis (harelip) -incomplete cleft–coloboma labii -cleft extending to the nose cavity - labium leporinum -cleft palate, soft palate - uranoschisis, jaw - gnatoschisis and uvula- staphyloschisis Medial, transverse,oblique cleft, Developmental disorders Vestibular defect Accesory mouth Macrostomy Microstomy Aglossia Microglossia Macroglossia Ankyloglossia Lingua plicata (scrotal tongue) Mikrognatia, progenia, prognatia Fordyce´s spots Dermoid cyst Heterotopic gastric and intestinal mucoses Heterotopic nerve tissue White nevus(white sponge n, AD inheritance intracellular edema Necrosis Mechanical Chemical (arsenic, acid, lye, drugs – aspirin) Scurvy (Scorbut) Hemoblastosis Infections Allergy (ATB, metals) Autoimmunity (Wegener granulomatosis, Lupus erytematodes….) Atrophy atrophy of mucosa and processus alveolares old age diabetes disorders of inervation, Plummer –Vinson‘s sy (atrophic glossitis + epithelial dysplasia in pernitious anemia anemia Pigmentation of the oral cavity melanin: physiological in darked races Hormonal: Graphite spots in Addison‘s disease melanin spots in Peutz-Jeghers syndrome Reactive: pigmentation in lichen planus actinic keratosis Therapy associated: Oral contraceptives Antimalarials Phenothiazines Methyldopa Pigmentation -

Congenital Epulis
Congenital Epulis Bernadette L. Koch, Charles Myer III, and John C. Egelhoff Summary: Congenital epulis of the newborn is a rare gingival surgeon. The infant was delivered without difficulty, with tumor that occurs along the alveolar ridge. We report the prena- Apgar score of 8/8/9 at 1, 5, and 10 minutes. The infant tal sonographic and postnatal MR imaging findings in an infant required no mechanical respiratory support, as the mass with maxillary and mandibular congenital epulides. did not obstruct the airway. After delivery, two separate Index terms: Temporomandibular joint, abnormalities and anom- pedunculated gingival masses were noted. One mass alies; Fetus, ultrasound arose from the anterior maxillary alveolar ridge and mea- sured 3 cm in greatest dimension; the second mass arose from the anterior mandibular alveolar ridge and measured The term congenital epulis of the newborn 2 cm (Fig 1B). The masses were well defined, smooth, and refers to a rare gingival tumor that most com- erythematous. The size and position of the masses caused monly occurs along the alveolar ridge of the superior deviation of the upper lip. Both nares were patent maxilla in newborn girls, usually without asso- with passage of an 8F catheter. The nose was flat and the ciated abnormalities of the teeth or additional nasal spine was absent, most likely as a result of deforma- congenital malformations. A case was de- tion from the gingival masses. Clinically, the osseous max- illary and mandibular ridges were thought to be normal. scribed by Neumann in 1871 (1). Since then, Postnatal MR imaging at 1 day of age revealed lobulat- multiple cases have been reported, primarily in ed masses that did not extend into the soft palate, floor of the pathologic, dental, and otolaryngologic lit- the mouth, nose, or cranium. -

Oral Mucosal Lesions in a Chilean Elderly Population: a Retrospective Study with a Systematic Review from Thirteen Countries
J Clin Exp Dent. 2017;9(2):e276-83. Oral mucosal lesions in elderly people Journal section: Oral Medicine and Pathology d oi:10.4317/jced. 53427 Publication Types: Research http://dx.doi.org/10.4317/jced.53427 Oral mucosal lesions in a Chilean elderly population: A retrospective study with a systematic review from thirteen countries César Rivera 1,2, Daniel Droguett 3, María-Jesús Arenas-Márquez 4 1 Department of Basic Biomedical Sciences, Faculty of Health Sciences, University of Talca (UTALCA), Talca, Chile 2 Department of Oral Diagnosis, School of Dentistry (FOP), University of Campinas (UNICAMP), Piracicaba, São Paulo, Brazil 3 Department of Stomatology, Faculty of Health Sciences, University of Talca (UTALCA), Talca, Chile 4 Gerontology Program, Faculty of Medical Sciences, University of Campinas (UNICAMP), Campinas, São Paulo, Brazil Correspondence: Jaime Rodríguez Carvajal Building University of Talca (UTALCA) Lircay Av. S/N, Talca, Chile Zip code 3460000 [email protected] Rivera C, Droguett D, Arenas-Márquez MJ. Oral mucosal lesions in a Chilean elderly population: A retrospective study with a systematic re- view from thirteen countries. J Clin Exp Dent. 2017;9(2):e276-83. Received: 12/08/2016 http://www.medicinaoral.com/odo/volumenes/v9i2/jcedv9i2p276.pdf Accepted: 22/08/2016 Article Number: 53427 http://www.medicinaoral.com/odo/indice.htm © Medicina Oral S. L. C.I.F. B 96689336 - eISSN: 1989-5488 eMail: [email protected] Indexed in: Pubmed Pubmed Central® (PMC) Scopus DOI® System Abstract Background: The oral examination is an essential part of the multidisciplinary medical care in elderly people. Oral mucosal lesions and normal variations of oral anatomy (OMLs) are very common in this people, but few studies have examined the frequency and prevalence of these conditions worldwide and less in Chile.