Pigmented Villonodular Synovitis of the Temporomandibular Joint: a Case Report and the Literature Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary for Narrative Writing
Periodontal Assessment and Treatment Planning Gingival description Color: o pink o erythematous o cyanotic o racial pigmentation o metallic pigmentation o uniformity Contour: o recession o clefts o enlarged papillae o cratered papillae o blunted papillae o highly rolled o bulbous o knife-edged o scalloped o stippled Consistency: o firm o edematous o hyperplastic o fibrotic Band of gingiva: o amount o quality o location o treatability Bleeding tendency: o sulcus base, lining o gingival margins Suppuration Sinus tract formation Pocket depths Pseudopockets Frena Pain Other pathology Dental Description Defective restorations: o overhangs o open contacts o poor contours Fractured cusps 1 ww.links2success.biz [email protected] 914-303-6464 Caries Deposits: o Type . plaque . calculus . stain . matera alba o Location . supragingival . subgingival o Severity . mild . moderate . severe Wear facets Percussion sensitivity Tooth vitality Attrition, erosion, abrasion Occlusal plane level Occlusion findings Furcations Mobility Fremitus Radiographic findings Film dates Crown:root ratio Amount of bone loss o horizontal; vertical o localized; generalized Root length and shape Overhangs Bulbous crowns Fenestrations Dehiscences Tooth resorption Retained root tips Impacted teeth Root proximities Tilted teeth Radiolucencies/opacities Etiologic factors Local: o plaque o calculus o overhangs 2 ww.links2success.biz [email protected] 914-303-6464 o orthodontic apparatus o open margins o open contacts o improper -

Clinical Features and Management of Oral Nonodontogenic Masses in Children
Clinical Features and Management of Oral Nonodontogenic Masses in Children Hao Zhang Nanjing Children’s Hospital of Nanjing Medical University Qiongqiong Zhou Nanjing Children’s Hospital of Nanjing Medical University Weimin Shen ( [email protected] ) Nanjing Children’s Hospital of Nanjing Medical University Research Article Keywords: Nonodontogenic masses, oral cavity, oral lesion, children, vascular anomalies, cystic masses, oral benign tumors Posted Date: December 11th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-122736/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/16 Abstract Background: There are numerous clinical reports of oral tumors in children. However, the clinical features and management of oral nonodontogenic masses in children were rare reported. The aim of this article is to present a large series of oral nonodontogenic masses in children, analyzing the clinical characteristics of such masses and reviewing the relevant procedures for treatment. Methods: We conducted an observational retrospective study, reviewing medical records of 171 patients who were treated for oral nonodontogenic masses between 2014 and 2019 at the Department of Pediatric Surgery, Children’s Hospital of Nanjing Medical University. Data collected included age, gender, site, pathologic diagnosis and treatment strategy. Results: All patients were hospitalized in our department. Of the 171 cases, all of them were benign, however, only 1 case diagnosed as inammatory myobroblastic tumor showed a malignant process. The most frequent type was hemangioma (63.7%), followed by lymphangioma (16.4%), ranula (7.6%). The most common location of oral masses in the buccal mucosa. The second common location was in tongue. -

Oral Diagnosis: the Clinician's Guide
Wright An imprint of Elsevier Science Limited Robert Stevenson House, 1-3 Baxter's Place, Leith Walk, Edinburgh EH I 3AF First published :WOO Reprinted 2002. 238 7X69. fax: (+ 1) 215 238 2239, e-mail: [email protected]. You may also complete your request on-line via the Elsevier Science homepage (http://www.elsevier.com). by selecting'Customer Support' and then 'Obtaining Permissions·. British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Library of Congress Cataloging in Publication Data A catalog record for this book is available from the Library of Congress ISBN 0 7236 1040 I _ your source for books. journals and multimedia in the health sciences www.elsevierhealth.com Composition by Scribe Design, Gillingham, Kent Printed and bound in China Contents Preface vii Acknowledgements ix 1 The challenge of diagnosis 1 2 The history 4 3 Examination 11 4 Diagnostic tests 33 5 Pain of dental origin 71 6 Pain of non-dental origin 99 7 Trauma 124 8 Infection 140 9 Cysts 160 10 Ulcers 185 11 White patches 210 12 Bumps, lumps and swellings 226 13 Oral changes in systemic disease 263 14 Oral consequences of medication 290 Index 299 Preface The foundation of any form of successful treatment is accurate diagnosis. Though scientifically based, dentistry is also an art. This is evident in the provision of operative dental care and also in the diagnosis of oral and dental diseases. While diagnostic skills will be developed and enhanced by experience, it is essential that every prospective dentist is taught how to develop a structured and comprehensive approach to oral diagnosis. -

A Single Case Report of Granular Cell Tumor of the Tongue Successfully Treated Through 445 Nm Diode Laser
healthcare Case Report A Single Case Report of Granular Cell Tumor of the Tongue Successfully Treated through 445 nm Diode Laser Maria Vittoria Viani 1,*, Luigi Corcione 1, Chiara Di Blasio 2, Ronell Bologna-Molina 3 , Paolo Vescovi 1 and Marco Meleti 1 1 Department of Medicine and Surgery, University of Parma, 43126 Parma, Italy; [email protected] (L.C.); [email protected] (P.V.); [email protected] (M.M.) 2 Private practice, Centro Medico Di Blasio, 43121 Parma; Italy; [email protected] 3 Faculty of Dentistry, University of the Republic, 14600 Montevideo, Uruguay; [email protected] * Correspondence: [email protected] Received: 10 June 2020; Accepted: 11 August 2020; Published: 13 August 2020 Abstract: Oral granular cell tumor (GCT) is a relatively rare, benign lesion that can easily be misdiagnosed. Particularly, the presence of pseudoepitheliomatous hyperplasia might, in some cases, lead to the hypothesis of squamous cell carcinoma. Surgical excision is the treatment of choice. Recurrence has been reported in up to 15% of cases treated with conventional surgery. Here, we reported a case of GCT of the tongue in a young female patient, which was successfully treated through 445 nm diode laser excision. Laser surgery might reduce bleeding and postoperative pain and may be associated with more rapid healing. Particularly, the vaporization effect on remnant tissues could eliminate GCT cells on the surgical bed, thus hypothetically leading to a lower rate of recurrence. In the present case, complete healing occurred in 1 week, and no recurrence was observed after 6 months. Laser surgery also allows the possibility to obtain second intention healing. -
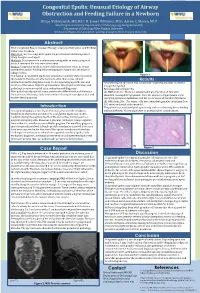
Congenital Epulis: Unusual Etiology of Airway Obstruction and Feeding Failure in a Newborn Shilpa Vishwanath, MD,MS1; H
Congenital Epulis: Unusual Etiology of Airway Obstruction and Feeding Failure in a Newborn Shilpa Vishwanath, MD,MS1; H. James Williams, MD2; Aaron C. Mason, MD3 1West Virginia University Department of Otolaryngology, Morgantown WV; 2Department of Pathology, West Virginia University 3Division of Plastic, Reconstructive, and Hand Surgery, West Virginia University Abstract Title: Congenital Epulis: Unusual Etiology of Airway Obstruction and Feeding Failure in a Newborn Objectives: Review congenital epulis; Its presentation and management. Study Design: Case Report Methods: Description of a newborn presenting with an obstructing oral mass. A review of the literature is included. Results: Congenital epulis is a rare oral lesion that may result in airway obstruction and/or feeding failure bringing the mass to the attention of subspecialists. Conclusion: A congenital epulis may present as a solitary alveolar mass in Figure 1 the newborn. Females are affected more often than males. Airway Results obstruction and feeding failure may evolve depending upon the size and The pathological specimen was a maxillary congenital granular cell tumor location of the lesion. Physical examination, radiographic evaluation, and (congenital epulis). pathologic review are useful in its evaluation and diagnosis. Pathology slides (Figure 3): Histopathologically, special stains assist in the differentiation of the lesion (A) H&E stain, 4x: There is a subepithelial proliferation of cells with from other solid tumors. Early intervention relieves airway obstruction and abundant eosinophilic cytoplasm. Note the absence of hyperplasia of the enables feeding success. overlying squamous epithelium and the prominence of vascular structures. (B) H&E stain, 20x: The tumor cells have abundant granular cytoplasm (low N/C ratio) and small uniform nuclei. -

Prevalence of Oral Lesions in Complete Denture Wearers- an Original Research
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 20, Issue 1 Ser.3 (January. 2021), PP 29-33 www.iosrjournals.org Prevalence of oral lesions in complete denture wearers- An original research Prenika Sharma1*, Reecha Gupta2 1- MDS, Oral medicine and radiology 2- Professor and HOD Department of Prosthodontics, Indira Gandhi Govt. Dental College, Jammu (J&K) Abstract: Background: Complete denture patients are often associated with the various denture-related oral mucosallesions. The purpose of this study is to evaluate the prevalence ofdenture-related oral mucosal lesions in complete denture patients. Materials and Methods: The study was consisted of 225 patientshaving various denture-induced oral mucosal lesions from theoutpatient department of the department out of the 395 completedenture patients examined. Data related to gender, age, length ofdenture use, hygiene care were obtained. All the data were tabulated and analyzed. Results: In 225 complete denture patients. Denture stomatitis (60.23%) was the most commonlesion present, followed by Epulis fissuratum and angularcheilitis. The denture-induced oral mucosal lesions werefound more common in age >40 years (59.78%) and in female(52.70%) complete denture wearer patients. Conclusion: The present studies showed that oral lesions associated with wearing denture are prevalent and create health problems that impact the quality of life of dental patients. Key Words: Complete denture, denture stomatitis, Epulis fissuratum, oralmucosal lesions. --------------------------------------------------------------------------------------------------------------------------------------- Date of Submission: 26-12-2020 Date of Acceptance: 07-01-2021 --------------------------------------------------------------------------------------------------------------------------------------- I. Introduction Edentulism may be the last sequel of periodontal diseases and dental caries. In case of older adults, edentulism is essential as a correlate of self-esteem and quality of life. -

Application of Lasers in Treatment of Oral Premalignant Lesions
Symbiosis www.symbiosisonline.org www.symbiosisonlinepublishing.com Review article Journal of Dentistry, Oral Disorders & Therapy Open Access Application of Lasers in Treatment of Oral Premalignant Lesions Amaninder Singh*1, Akanksha Zutshi2, Preetkanwal Singh Ahluwalia3, Vikas Sharma4 and Vandana Razdan5 1,4oral and maxillofacial surgery, reader, National Dental College and Hospital, Dera Bassi, Punjab 2oral and maxillofacial surgery, senior lecturer, National Dental College and Hospital, Dera Bassi, Punjab 3oral and maxillofacial surgery, professor, National Dental College and Hospital, Dera Bassi, Punjab 5Pharmacology, professor, Govt. Medical College and Hospital, Jammu Received: April 03, 2018; Accepted: June 04, 2018; Published: June 11, 2018 *Corresponding author: Amaninder Singh, House No- 620, Phase- 6, mohali, 160055, E-mail address: [email protected] Abstract radiation. Laser systems and their application in dentistry and especially the basis of energy of the beam and wavelength of the emitted oral surgery are rapidly improving today. Lasers are being used as a niche tool as direct replacement for conventional approaches ClassificationGas lasers of lasers [6] CO advantages of lasers are incision of tissues, coagulation during Argon like scalpel, blades, electro surgery, dental hand piece. The specific Liquid Dyes2 canoperation be used and for treatmentpostoperative of conditions benefits likesuch lowas premalignant postoperative lesions, pain, better wound healing. For soft tissue oral surgical procedures lasers Solid -

Supernumerary Teeth in Primary Dentition Associated to Palatal Polyps
Revista Odontológica Mexicana Facultad de Odontología Vol. 17, No. 3 July-September 2013 pp 168-172 CASE REPORT Supernumerary teeth in primary dentition associated to palatal polyps. Case report Dientes supernumerarios en dentición primaria asociados a pólipos palatinos. Reporte de caso Thalia Sánchez Muñoz Ledo,* Alejandro Hinojosa Aguirre,§ Germán Portillo Guerrero,II Fernando Tenorio Rocha¶ ABSTRACT RESUMEN Polyps are rare in children. The present article reports the clinical Los pólipos son poco frecuentes en niños. En este artículo se pre- case of a 14 month old male patient brought for treatment to the senta un caso clínico de un niño de un año dos meses que acude Pedodontics Clinic of the Graduate School, National School of a la Clínica de Odontopediatría de la DEPeI UNAM con dos póli- Dentistry National University of Mexico. He presented two palatal pos fibroepiteliales palatinos ubicados a ambos lados de la papila fibro-epithelial polyps, located at both sides of the incisive papilla. incisiva, 10 días posteriores a la excisión quirúrgica se observó la 10 days after surgical excision, a supernumerary tooth erupted in erupción de un diente supernumerario en el paladar, y 25 días des- the palate. 25 days later, eruption of a second supernumerary tooth pués se observó la erupción de un segundo diente supernumerario. was observed. Both teeth were extracted. Histological diagnosis Ambos dientes fueron extraídos. El diagnóstico histológico de las of palatal lesions was giant fibroblast fibroma. Nevertheless, no lesiones en el paladar fue: fibroma de fibroblastos gigantes; sin -em histological evidence was found to show possible relationship bargo, no se encontró evidencia histológica que mostrara alguna between presence of palatal polyps and supernumerary teeth. -

Copyrighted Material
Part 1 General Dermatology GENERAL DERMATOLOGY COPYRIGHTED MATERIAL Handbook of Dermatology: A Practical Manual, Second Edition. Margaret W. Mann and Daniel L. Popkin. © 2020 John Wiley & Sons Ltd. Published 2020 by John Wiley & Sons Ltd. 0004285348.INDD 1 7/31/2019 6:12:02 PM 0004285348.INDD 2 7/31/2019 6:12:02 PM COMMON WORK-UPS, SIGNS, AND MANAGEMENT Dermatologic Differential Algorithm Courtesy of Dr. Neel Patel 1. Is it a rash or growth? AND MANAGEMENT 2. If it is a rash, is it mainly epidermal, dermal, subcutaneous, or a combination? 3. If the rash is epidermal or a combination, try to define the SIGNS, COMMON WORK-UPS, characteristics of the rash. Is it mainly papulosquamous? Papulopustular? Blistering? After defining the characteristics, then think about causes of that type of rash: CITES MVA PITA: Congenital, Infections, Tumor, Endocrinologic, Solar related, Metabolic, Vascular, Allergic, Psychiatric, Latrogenic, Trauma, Autoimmune. When generating the differential, take the history and location of the rash into account. 4. If the rash is dermal or subcutaneous, then think of cells and substances that infiltrate and associated diseases (histiocytes, lymphocytes, mast cells, neutrophils, metastatic tumors, mucin, amyloid, immunoglobulin, etc.). 5. If the lesion is a growth, is it benign or malignant in appearance? Think of cells in the skin and their associated diseases (keratinocytes, fibroblasts, neurons, adipocytes, melanocytes, histiocytes, pericytes, endothelial cells, smooth muscle cells, follicular cells, sebocytes, eccrine -

Research Papers Published in Pubmed Indexed Journals
List of Reseach papers published in PubMed Indexed Journals during the last five years Link of the recognition in Department of the Year of Name of the S.No Title of the Paper Name of the author/s Name of the Journal ISSN NUMBER UGC enlistment of the teacher Publication indexing database journal Comparative evaluation of the effect of menstruation, pregnancy and Saluja P, Shetty VP, Dave A, Arora M, Hans https://www.ncbi.nlm.nih.gov/pub 1 oral Pathology Journal of Clinical & Diagnostics Research 2014 0973-709X PubMed menopause on salivary flow rate, ph and gustatory function. V, Madan A med/25478455 JOURNAL OF INTERNATIONAL SOCIETY Comparison of root canal sealer distribution in obturated root canal-An Conservative Dentistry And https://www.ncbi.nlm.nih.gov/pm 2 SetyaG OF PREVENTIVE AND COMMUNITY 2014 22310762 PubMed in-vitro study Endododntics c/articles/PMC4209620/ DENTISTRY Comparison of the anaesthetic efficacy of epinephrine concentrations (1 : 80 000 and 1 : 200 000) in 2% lidocaine for inferior alveolar nerve Aggarwal, V., Singla, M., Miglani, S., Kohli, Conservative Dentistry And https://www.ncbi.nlm.nih.gov/pub 3 International endodontic journal 2014 0143-2885 PubMed block in patients with symptomatic irreversible pulpitis: A randomized, S. Endododntics med/23895176 double-blind clinical tria The effect of ferrule presence and type of dowel on fracture resistance of Aggarwal, V., Singla, M., Yadav, S.Sharma, Conservative Dentistry And https://www.ncbi.nlm.nih.gov/pub 4 Journal of Conservative Dentistqry 2014 0972-0707 PubMed endodontically treated teeth restored with metal-ceramic crowns V., Bhasin, S.S. -

The Peripheral Giant Cell Granuloma in Edentulous Patients: Report of Three Unique Cases
Published online: 2019-09-30 The Peripheral Giant Cell Granuloma in Edentulous Patients: Report of Three Unique Cases Osman A. Etoza Ahmet Emin Demirbasa Mehmet Bulbulb Ebru Akayc ABSTRACT The peripheral giant cell granuloma (PGCG) is a rare reactive exophytic lesion taking place on the gingiva and alveolar ridge usually as a result of local irritating factors such as trauma, tooth extrac- tion, badly finished fillings, unstable dental prosthesis, plaque, calculus, chronic infections, and im- pacted food. This article presents 3 cases of PGCG that presented at the same location of the edentu- lous mandible of patients that using complete denture for over ten years. (Eur J Dent 2010;4:329-333) Key words: Peripheral giant cell granuloma; Chronic irritation; Edentulous patients; Complete denture. INTRODUCTION Giant cell granuloma lesions (peripheral and teoclastoma, or giant-cell hyperplasia. Etiologic central) are benign, non-odontogenic, moderately factors are not known, although it is thought that rare tumors of the oral cavity. They develop pe- it may be due to an irritant or aggressive factor ripherally (within gingiva) or centrally (in bone).1 such as trauma, tooth extraction, badly finished The peripheral giant cell granuloma (PGCG) is a fillings, unstable dental prosthesis, plaque, calcu- rare reactive exophytic lesion taking place on the lus, chronic infections, or impacted food.2,3 Clini- gingiva and alveolar ridge, also known as a giant- cal appearance of PGCGs can present as polyploi- cell epulis, giant-cell reparative granuloma, os- dy or nodular lesions, primarily bluish red with a smooth shiny or mamillated surface, stalky or 2,4,5 a Erciyes University, Faculty of Dentistry, Department of sessile base, small and well demarcated. -

Treatments for Ankyloglossia and Ankyloglossia with Concomitant Lip-Tie Comparative Effectiveness Review Number 149
Comparative Effectiveness Review Number 149 Treatments for Ankyloglossia and Ankyloglossia With Concomitant Lip-Tie Comparative Effectiveness Review Number 149 Treatments for Ankyloglossia and Ankyloglossia With Concomitant Lip-Tie Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. 290-2012-00009-I Prepared by: Vanderbilt Evidence-based Practice Center Nashville, TN Investigators: David O. Francis, M.D., M.S. Sivakumar Chinnadurai, M.D., M.P.H. Anna Morad, M.D. Richard A. Epstein, Ph.D., M.P.H. Sahar Kohanim, M.D. Shanthi Krishnaswami, M.B.B.S., M.P.H. Nila A. Sathe, M.A., M.L.I.S. Melissa L. McPheeters, Ph.D., M.P.H. AHRQ Publication No. 15-EHC011-EF May 2015 This report is based on research conducted by the Vanderbilt Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 290-2012-00009-I). The findings and conclusions in this document are those of the authors, who are responsible for its contents; the findings and conclusions do not necessarily represent the views of AHRQ. Therefore, no statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. The information in this report is intended to help health care decisionmakers—patients and clinicians, health system leaders, and policymakers, among others—make well-informed decisions and thereby improve the quality of health care services. This report is not intended to be a substitute for the application of clinical judgment.