A Single Case Report of Granular Cell Tumor of the Tongue Successfully Treated Through 445 Nm Diode Laser
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary for Narrative Writing
Periodontal Assessment and Treatment Planning Gingival description Color: o pink o erythematous o cyanotic o racial pigmentation o metallic pigmentation o uniformity Contour: o recession o clefts o enlarged papillae o cratered papillae o blunted papillae o highly rolled o bulbous o knife-edged o scalloped o stippled Consistency: o firm o edematous o hyperplastic o fibrotic Band of gingiva: o amount o quality o location o treatability Bleeding tendency: o sulcus base, lining o gingival margins Suppuration Sinus tract formation Pocket depths Pseudopockets Frena Pain Other pathology Dental Description Defective restorations: o overhangs o open contacts o poor contours Fractured cusps 1 ww.links2success.biz [email protected] 914-303-6464 Caries Deposits: o Type . plaque . calculus . stain . matera alba o Location . supragingival . subgingival o Severity . mild . moderate . severe Wear facets Percussion sensitivity Tooth vitality Attrition, erosion, abrasion Occlusal plane level Occlusion findings Furcations Mobility Fremitus Radiographic findings Film dates Crown:root ratio Amount of bone loss o horizontal; vertical o localized; generalized Root length and shape Overhangs Bulbous crowns Fenestrations Dehiscences Tooth resorption Retained root tips Impacted teeth Root proximities Tilted teeth Radiolucencies/opacities Etiologic factors Local: o plaque o calculus o overhangs 2 ww.links2success.biz [email protected] 914-303-6464 o orthodontic apparatus o open margins o open contacts o improper -

Skin and Breast Disease in the Differential Diagnosis of Chest Pain
Skin and breast disease in the differential diagnosis of chest pain Author Muir, Jim, Yelland, Michael Published 2010 Journal Title Medical Clinics of North America DOI https://doi.org/10.1016/j.mcna.2010.01.006 Copyright Statement © 2010 Elsevier. This is the author-manuscript version of this paper. Reproduced in accordance with the copyright policy of the publisher. Please refer to the journal's website for access to the definitive, published version. Downloaded from http://hdl.handle.net/10072/33712 Griffith Research Online https://research-repository.griffith.edu.au ARTICLE IN PRESS 1 2 Skin and Breast 3 4 Disease in the 5 6 Differential 7 8 Diagnosis of 9 10 Chest Pain 11 12 a, b ½Q2½ Q3 Jim Muir *, Michael Yelland ½Q4½ Q5 KEYWORDS 13 14 Chest pain Skin diseases Herpes zoster PROOF 15 Breast Neoplasm 16 17 18 Pain is not a symptom commonly associated with skin disease. This is especially so 19 when considering the known skin problems that have a presenting symptom of chest 20 pain that could potentially be confused with chest pain from other causes. 21 22 PAINFUL SKIN CONDITIONS 23 24 Several extremely painful and tender skin conditions present with dramatic clinical 25 signs. Inflammatory disorders such as pyoderma gangrenosum, skin malignancies, 26 both primary and secondary, acute bacterial infections such as erysipelas or cellulitis, 27 and multiple other infections are commonly extremely painful and tender. As these 28 conditions manifest with obvious skin signs such as swelling, erythema, localized 29 tenderness, fever, lymphangitis, and lymphadenopathy, there is little chance of misdi- 30 agnosis of symptoms as caused by anything other than a cutaneous pathology. -

Oral Diagnosis: the Clinician's Guide
Wright An imprint of Elsevier Science Limited Robert Stevenson House, 1-3 Baxter's Place, Leith Walk, Edinburgh EH I 3AF First published :WOO Reprinted 2002. 238 7X69. fax: (+ 1) 215 238 2239, e-mail: [email protected]. You may also complete your request on-line via the Elsevier Science homepage (http://www.elsevier.com). by selecting'Customer Support' and then 'Obtaining Permissions·. British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Library of Congress Cataloging in Publication Data A catalog record for this book is available from the Library of Congress ISBN 0 7236 1040 I _ your source for books. journals and multimedia in the health sciences www.elsevierhealth.com Composition by Scribe Design, Gillingham, Kent Printed and bound in China Contents Preface vii Acknowledgements ix 1 The challenge of diagnosis 1 2 The history 4 3 Examination 11 4 Diagnostic tests 33 5 Pain of dental origin 71 6 Pain of non-dental origin 99 7 Trauma 124 8 Infection 140 9 Cysts 160 10 Ulcers 185 11 White patches 210 12 Bumps, lumps and swellings 226 13 Oral changes in systemic disease 263 14 Oral consequences of medication 290 Index 299 Preface The foundation of any form of successful treatment is accurate diagnosis. Though scientifically based, dentistry is also an art. This is evident in the provision of operative dental care and also in the diagnosis of oral and dental diseases. While diagnostic skills will be developed and enhanced by experience, it is essential that every prospective dentist is taught how to develop a structured and comprehensive approach to oral diagnosis. -

ROLE of MAST CELL in ORAL PATHOLOGY Supriya Kheur Deepali Patekar Neeta Bagul Meena Kulkarni Samapika Routray 1 Varsha Dhas Department of Oral Pathology,Dr
320 ROLE OF MAST CELL IN ORAL PATHOLOGY Supriya Kheur Deepali Patekar Neeta Bagul Meena Kulkarni Samapika Routray 1 Varsha Dhas Department of Oral Pathology,Dr. D.Y.Patil Dental College and Hospital, Pimpri, Pune-18, India 1Department of Oral Pathology, GITAM Dental College, Vishakhapattanam, India Corresponding Author: Supriya Kheur, Department of Oral Pathology,Dr. D.Y.Patil Dental College and Hospital, Pimpri, Pune-18, India. Ph -09970150760, Email : [email protected] Abstract Mast cells in oral tissues releases various pro-inflammatory cytokine tumor necrosis factor alpha (TNF-Ü) which promotes leukocyte infiltration in various inflammatory condition of oral cavity such as oral lichen planus (OLP), periapical lesions, gingivitis & periodontitis. T lymphocyte derived cytokines influences mast cell migration & mediator release. Mast cell secreted proteases, activates matrix-metalloprotinases-9 (MMP-9) which may contribute to alteration in basement membrane in inflammatory condition such as Lichen Planus. Hence by understanding the role of mast cells in the pathogenesis of various diseases; therapies should be targeted to enhance the prognosis of the diseases. Key Words: Mast cells, Degranulation, Cytokines, Tryptase, Chymase. Introduction chemotactins, cell activating & cell growth Mast cells (MC) are large spherical or factor. Tissue mast cells are not homogenous elliptical mononuclear cells found in a variety for eg. enzymes within granules of mucosal & of tissues including skin, submucosa or connective tissue mast cells are different from connective tissue of various organs & each other. The ranges of mast cell activity is (1) specialized for their anatomic location, as the mucosal epithelial tissues & also in dental granules are different for mucosal and pulp. -

Rush University Medical Center, May 2005
TABLE OF CONTENTS Case # Title Page 1. Malignant Spitz’s Nevus 1 2. Giant Congenital Nevus 4 3. Methotrexate Nodulosis 7 4. Apthae with Trisomy 8–positive Myelodysplastic Syndrome 10 5. Kwashiorkor 13 6. “Unknown” 16 7. Gangrenous Cellulitis 17 8. Parry-Romberg Syndrome 21 9. Wegener’s Granulomatosis 24 10. Pediatric CTCL 27 11. Hypopigmented Mycosis Fungoides 30 12. Fabry’s Disease 33 13. Cicatricial Alopecia, Unclassified 37 14. Mastocytoma 40 15. Cutaneous Piloleiomyomas 42 16. Granular Cell Tumor 44 17. Disseminated Blastomycoses 46 18. Neonatal Lupus 49 19. Multiple Lipomas 52 20. Acroangiodermatitis of Mali 54 21. Pigmented Basal Cell Carcinoma (BCC) 57 Page 1 Case #1 CHICAGO DERMATOLOGICAL SOCIETY RUSH UNIVERSITY MEDICAL CENTER CHICAGO, ILLINOIS MAY 18, 2005 CASE PRESENTED BY: Michael D. Tharp, M.D. Lady Dy, M.D., and Darrell W. Gonzales, M.D. History: This 2 year-old white female presented with a one year history of an expanding lesion on her left cheek. There was no history of preceding trauma. The review of systems was normal. Initially the lesion was thought to be a pyogenic granuloma and treated with two courses of pulse dye laser. After no response to treatment, a shave biopsy was performed. Because the histopathology was interpreted as an atypical melanocytic proliferation with Spitzoid features, a conservative, but complete excision with margins was performed. The pathology of this excision was interpreted as malignant melanoma measuring 4.0 mm in thickness. A sentinel lymph node biopsy was subsequently performed and demonstrated focal spindle cells within the subcapsular sinus of a left preauricular lymph node. -

Benign Tumors and Tumor-Like Lesions of the Vulva
Please do not remove this page Benign Tumors and Tumor-like Lesions of the Vulva Heller, Debra https://scholarship.libraries.rutgers.edu/discovery/delivery/01RUT_INST:ResearchRepository/12643402930004646?l#13643525330004646 Heller, D. (2015). Benign Tumors and Tumor-like Lesions of the Vulva. In Clinical Obstetrics & Gynecology (Vol. 58, Issue 3, pp. 526–535). Rutgers University. https://doi.org/10.7282/T3RN3B2N This work is protected by copyright. You are free to use this resource, with proper attribution, for research and educational purposes. Other uses, such as reproduction or publication, may require the permission of the copyright holder. Downloaded On 2021/09/23 14:56:57 -0400 Heller DS Benign Tumors and Tumor-like lesions of the Vulva Debra S. Heller, MD From the Department of Pathology & Laboratory Medicine, Rutgers-New Jersey Medical School, Newark, NJ Address Correspondence to: Debra S. Heller, MD Dept of Pathology-UH/E158 Rutgers-New Jersey Medical School 185 South Orange Ave Newark, NJ, 07103 Tel 973-972-0751 Fax 973-972-5724 [email protected] Funding: None Disclosures: None 1 Heller DS Abstract: A variety of mass lesions may affect the vulva. These may be non-neoplastic, or represent benign or malignant neoplasms. A review of benign mass lesions and neoplasms of the vulva is presented. Key words: Vulvar neoplasms, vulvar diseases, vulva 2 Heller DS Introduction: A variety of mass lesions may affect the vulva. These may be non-neoplastic, or represent benign or malignant neoplasms. Often an excision is required for both diagnosis and therapy. A review of the more commonly encountered non-neoplastic mass lesions and benign neoplasms of the vulva is presented. -
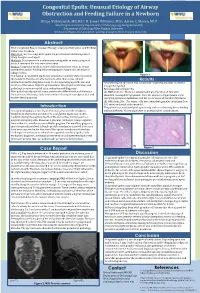
Congenital Epulis: Unusual Etiology of Airway Obstruction and Feeding Failure in a Newborn Shilpa Vishwanath, MD,MS1; H
Congenital Epulis: Unusual Etiology of Airway Obstruction and Feeding Failure in a Newborn Shilpa Vishwanath, MD,MS1; H. James Williams, MD2; Aaron C. Mason, MD3 1West Virginia University Department of Otolaryngology, Morgantown WV; 2Department of Pathology, West Virginia University 3Division of Plastic, Reconstructive, and Hand Surgery, West Virginia University Abstract Title: Congenital Epulis: Unusual Etiology of Airway Obstruction and Feeding Failure in a Newborn Objectives: Review congenital epulis; Its presentation and management. Study Design: Case Report Methods: Description of a newborn presenting with an obstructing oral mass. A review of the literature is included. Results: Congenital epulis is a rare oral lesion that may result in airway obstruction and/or feeding failure bringing the mass to the attention of subspecialists. Conclusion: A congenital epulis may present as a solitary alveolar mass in Figure 1 the newborn. Females are affected more often than males. Airway Results obstruction and feeding failure may evolve depending upon the size and The pathological specimen was a maxillary congenital granular cell tumor location of the lesion. Physical examination, radiographic evaluation, and (congenital epulis). pathologic review are useful in its evaluation and diagnosis. Pathology slides (Figure 3): Histopathologically, special stains assist in the differentiation of the lesion (A) H&E stain, 4x: There is a subepithelial proliferation of cells with from other solid tumors. Early intervention relieves airway obstruction and abundant eosinophilic cytoplasm. Note the absence of hyperplasia of the enables feeding success. overlying squamous epithelium and the prominence of vascular structures. (B) H&E stain, 20x: The tumor cells have abundant granular cytoplasm (low N/C ratio) and small uniform nuclei. -

Subcutaneous Hemangiosarcoma: the First Report in Maltese Dog
pISSN 1598-298X / eISSN 2384-0749 J Vet Clin 36(3) : 169-171 (2019) http://dx.doi.org/10.17555/jvc.2019.06.36.3.169 Subcutaneous Hemangiosarcoma: The First Report in Maltese Dog Ha-Jung Kim, Eun-Taek Hong and Guk-Hyun Suh1 Department of Veterinary Internal Medicine, College of Veterinary Medicine, Chonnam National University, Gwangju 500-757, Korea (Received: March 13, 2019 / Accepted: May 09, 2019) Abstract : Subcutanous hemangiosarcoma is rare malignant condition in dogs. An eleven-year-old neutered male Maltese was presented with multicentric cutaneous hemorrhagic nodules followed by lethargy. The patient showed regenerative anemia and thrombocytopenia with skyrocketing D-dimer, indicating that he had disseminated intravascular coagulation (DIC) on progress. Fine needle aspiration, histopathology, X-ray, and computed tomographic scanning ultimately diagnosed this patient as subcutaneous hemangiosarcoma with disseminated metastasis to the body. Unfortunately, the dog died due to side effects of anti-thrombotic therapy for DIC. This case report described a rare subcutaneous hemangiosarcoma in a Maltese dog. Key words : dog, skin neoplasms, hemangiosarcoma, disseminated intravascular coagulation, histopathology. Introduction had a 2 month history of multicentric cutaneous hemor- rhagic nodules initiated from his dorsum (Fig 1A and C). Hemangiosarcoma (a.k.a. malignant hemangioendotheli- There were no specific findings from skin examination such oma or angisarcoma) is an outbreak of tumor from endothe- as scraping or taping, and no bacteria or fungi were cultured lial cells which occurs more frequently in dogs than any from the lesions. On physical examination, he had a pale other species, accounting for 0.3% to 2.0% of all tumors in mucous membrane with bilateral ocular hemorrhage (Fig dogs with a high fatality rate (1,7). -

Atypical Fibroids
Hereditary leiomyomatosis and renal cell cancer: Cutaneous lesions & atypical fibroids The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Bortoletto, Pietro, Jennifer L. Lindsey, Liping Yuan, Bradley J. Quade, Antonio R. Gargiulo, Cynthia C. Morton, Elizabeth A. Stewart, and Raymond M. Anchan. 2017. “Hereditary leiomyomatosis and renal cell cancer: Cutaneous lesions & atypical fibroids.” Case Reports in Women's Health 15 (1): 31-34. doi:10.1016/ j.crwh.2017.06.004. http://dx.doi.org/10.1016/j.crwh.2017.06.004. Published Version doi:10.1016/j.crwh.2017.06.004 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:35982080 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Case Reports in Women's Health 15 (2017) 31–34 Contents lists available at ScienceDirect Case Reports in Women's Health journal homepage: www.elsevier.com/locate/crwh Hereditary leiomyomatosis and renal cell cancer: Cutaneous MARK lesions & atypical fibroids Pietro Bortolettoa,b,c,1, Jennifer L. Lindseya,b,1, Liping Yuand, Bradley J. Quadec,d, Antonio R. Gargiuloa,b,c, Cynthia C. Mortonb,c,d,e,f, Elizabeth A. Stewartg, ⁎ Raymond M. Anchana,b,c, a Division of Reproductive Endocrinology and Infertility, Boston, MA, USA b Department of Obstetrics, Gynecology and Reproductive Biology, -

Growing Papule on the Right Shoulder of an Elderly Man
DERMATOPATHOLOGY DIAGNOSIS CLOSE ENCOUNTERS WITH THE ENVIRONMENT Growing Papule on the Right Shoulder of an Elderly Man Campbell L. Stewart, MD; Karolyn A. Wanat, MD; Adam I. Rubin, MD copy A not B H&E, original magnification ×20. H&E, original magnification ×400. Do The best diagnosis is: a. desmoplastic trichilemmoma b. granular cell basal cell carcinoma c. granular cell tumor d. sebaceous adenoma CUTIS e. xanthogranuloma PLEASE TURN TO PAGE 392 FOR DERMATOPATHOLOGY DIAGNOSIS DISCUSSION Dr. Stewart is from the University of Washington, Seattle. Dr. Wanat is from the University of Iowa, Iowa City. Dr. Rubin is from the University of Pennsylvania, Philadelphia. The authors report no conflict of interest. Correspondence: Adam I. Rubin, MD, University of Pennsylvania, 2 Maloney Bldg, 3600 Spruce St, Philadelphia, PA 19104 ([email protected]). WWW.CUTIS.COM VOLUME 97, JUNE 2016 391 Copyright Cutis 2016. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. Dermatopathology Diagnosis Discussion Granular Cell Basal Cell Carcinoma asal cell carcinoma (BCC) is the most com- The granules in GBCC generally are positive on peri- mon human epithelial malignancy. There are odic acid–Schiff staining.1-4 several histologic variants, the rarest being The histologic differential diagnosis for GBCC B 1 granular cell BCC (GBCC). Granular cell BCC is includes granular cell tumor as well as other tumors reported most commonly in men with a mean age that can present with granular cell changes such as of 63 years. Sixty-four percent of cases develop on ameloblastoma, leiomyoma, leiomyosarcoma, angio- the face, with the remainder arising on the chest sarcoma, malignant peripheral nerve sheath tumor, or trunk. -

Download Download
628 Indian Journal of Forensic Medicine & Toxicology, July-September 2021, Vol. 15, No. 3 Tongue Lesions - A Review N.Anitha1, Dharini Jayachandran2 1Reader, Department of Oral Pathology and Microbiology,2Undergraduate Student, Sree Balaji Dental College and Hospital, Bharath Institute of Higher Education and Research Abstract Tongue is a vital organ within the oral cavity that has varied function,and it may act as an index for the underlying systemic diseases.The investigation of the tongue diseases may begin with mere clinical examination .This review is to highlight the signs and symptoms of the various lesions that affects the tongue and especially to talk in brief about the benign and malignant tumours that affect the tongue along with other inherited and congenital abnormalities.Tongue lesions are categorized as tumours,infections, reactionary,congenital,developmental,acquired,autoimmune and potentially malignant disorders for easy understanding and to arrive at appropriate diagnosis.Tongue playing an important role in maintaining the harmony in the oral environment,it should be treated from diseases. Keywords: Tongue lesions,benign tumours,malignant tumours,diseases of tongue. CLASSIFICATION OF LESIONS ● Pyogenic granuloma AFFECTING THE TONGUE. ● Frictional keratosis BENIGN TUMOURS OF THE TONGUE INFECTIOUS LESIONS OF TONGUE ● Capillary hemangioma ● Oral squamous papilloma ● Fibroma ● Oral hairy leukoplakia ● Cavernous hemangioma ● Candidiasiis ● Giant cell granuloma ● Median rhomboid glossitis ● Lipoma ● Sublingual abcess ● Lymphangioma INHERITED,CONGENITAL,DEVELOPMENT ● Schwannoma AND ACQUIRED ABNORMALITIES OF TONGUE MALIGNANT TUMOURS OF TONGUE ● White sponge nevus ● Squamous cell carcinoma ● Foliate papillitis ● Veruccous carcinoma ● Angina bullosa hemorrhagica ● Non-Hodgkin’s lymphoma ● Geographic tongue TRAUMATIC/REACTIONARY LESIONS OF ● Fissured tongue THE TONGUE ● Median rhomboid glossitis ● Fibrous reactive hyperplasia ● Bifurcated/tetrafurcated tongue ● Traumatic ulcer Indian Journal of Forensic Medicine & Toxicology, July-September 2021, Vol. -

Pyogenic Granuloma on the Upper Lip: an Unusual Location
www.scielo.br/jaos Pyogenic granuloma on the upper lip: an unusual location Eduardo Sanches GONÇALES1, José Humberto DAMANTE2, Cassia Maria FISCHER RUBIRA3, Luís Antônio de Assis Taveira4 1- DDS, PhD, Assistant Professor, Department of Stomatology, Bauru School of Dentistry, University of São Paulo, Bauru, SP, Brazil. 2- DDS, PhD, Professor, Department of Stomatology, Bauru School of Dentistry, University of São Paulo, Bauru, SP, Brazil. 3- DDS, PhD in Stomatology, Bauru School of Dentistry, University of São Paulo, Bauru, SP, Brazil. 4- DDS, PhD, Associate Professor, Department of Stomatology, Bauru School of Dentistry, University of São Paulo, Bauru, SP, Brazil. Corresponding address: Eduardo Sanches Gonçales - Faculdade de Odontologia de Bauru - USP - Departmento de Estomatologia - Al. Dr. Octavio Pinheiro Brisolla 9-75 - 17.012-901 - Bauru, SP - Brasil - Phone: 55 14 32358250 - e-mail:[email protected] Received: March 23, 2009 - Modification: September 16, 2009 - Accepted: October 11, 2009 ABSTRACT yogenic granuloma (PG) is a benign non-neoplastic mucocutaneous lesion. It is a Preactional response to constant minor trauma and might be related to hormonal changes. In the mouth, PG is manifested as a sessile or pedunculated, resilient, erythematous, exophytic and painful papule or nodule with a smooth or lobulated surface that bleeds easily. PG preferentially affects the gingiva, but may also occur on the lips, tongue, oral mucosa and palate. The most common treatment is surgical excision. This paper describes a mucocutaneous PG on the upper lip, analyzing the clinical characteristics and discussing the features that distinguish this lesion from other similar oral mucosa lesions. The diagnosis of oral lesions is complex and leads the dentist to consider distinct lesions with different diagnostic methods.