Pharmacy on the Isle of Wight ‘A Guide to Pharmacy & Pharmacy Services’
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
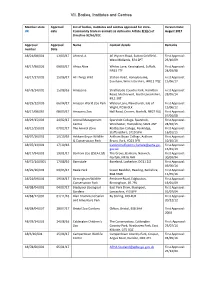
VII. Bodies, Institutes and Centres
VII. Bodies, Institutes and Centres Member state Approval List of bodies, institutes and centres approved for intra- Version Date: UK date Community trade in animals as defined in Article 2(1)(c) of August 2017 Directive 92/65/EEC Approval Approval Name Contact details Remarks number Date AB/21/08/001 13/03/17 Ahmed, A 46 Wyvern Road, Sutton Coldfield, First Approval: West Midlands, B74 2PT 23/10/09 AB/17/98/026 09/03/17 Africa Alive Whites Lane, Kessingland, Suffolk, First Approval: NR33 7TF 24/03/98 AB/17/17/005 15/06/17 All Things Wild Station Road, Honeybourne, First Approval: Evesham, Worcestershire, WR11 7QZ 15/06/17 AB/78/14/002 15/08/16 Amazonia Strathclyde Country Park, Hamilton First Approval: Road, Motherwell, North Lanarkshire, 28/05/14 ML1 3RT AB/29/12/003 06/04/17 Amazon World Zoo Park Watery Lane, Newchurch, Isle of First Approval: Wight, PO36 0LX 15/06/12 AB/17/08/065 08/03/17 Amazona Zoo Hall Road, Cromer, Norfolk, NR27 9JG First Approval: 07/04/08 AB/29/15/003 24/02/17 Animal Management Sparsholt College, Sparsholt, First Approval: Centre Winchester, Hampshire, SO21 2NF 24/02/15 AB/12/15/001 07/02/17 The Animal Zone Rodbaston College, Penkridge, First Approval: Staffordshire, ST19 5PH 16/01/15 AB/07/16/001 10/10/16 Askham Bryan Wildlife Askham Bryan College, Askham First Approval: & Conservation Park Bryan, York, YO23 3FR 10/10/16 AB/07/13/001 17/10/16 [email protected]. First Approval: gov.uk 15/01/13 AB/17/94/001 19/01/17 Banham Zoo (ZSEA Ltd) The Grove, Banham, Norwich, First Approval: Norfolk, NR16 -

Verzeichnis Der Europäischen Zoos Arten-, Natur- Und Tierschutzorganisationen
uantum Q Verzeichnis 2021 Verzeichnis der europäischen Zoos Arten-, Natur- und Tierschutzorganisationen Directory of European zoos and conservation orientated organisations ISBN: 978-3-86523-283-0 in Zusammenarbeit mit: Verband der Zoologischen Gärten e.V. Deutsche Tierpark-Gesellschaft e.V. Deutscher Wildgehege-Verband e.V. zooschweiz zoosuisse Schüling Verlag Falkenhorst 2 – 48155 Münster – Germany [email protected] www.tiergarten.com/quantum 1 DAN-INJECT Smith GmbH Special Vet. Instruments · Spezial Vet. Geräte Celler Str. 2 · 29664 Walsrode Telefon: 05161 4813192 Telefax: 05161 74574 E-Mail: [email protected] Website: www.daninject-smith.de Verkauf, Beratung und Service für Ferninjektionsgeräte und Zubehör & I N T E R Z O O Service + Logistik GmbH Tranquilizing Equipment Zootiertransporte (Straße, Luft und See), KistenbauBeratung, entsprechend Verkauf undden Service internationalen für Ferninjektionsgeräte und Zubehör Vorschriften, Unterstützung bei der Beschaffung der erforderlichenZootiertransporte Dokumente, (Straße, Vermittlung Luft und von See), Tieren Kistenbau entsprechend den internationalen Vorschriften, Unterstützung bei der Beschaffung der Celler Str.erforderlichen 2, 29664 Walsrode Dokumente, Vermittlung von Tieren Tel.: 05161 – 4813192 Fax: 05161 74574 E-Mail: [email protected] Str. 2, 29664 Walsrode www.interzoo.deTel.: 05161 – 4813192 Fax: 05161 – 74574 2 e-mail: [email protected] & [email protected] http://www.interzoo.de http://www.daninject-smith.de Vorwort Früheren Auflagen des Quantum Verzeichnis lag eine CD-Rom mit der Druckdatei im PDF-Format bei, welche sich großer Beliebtheit erfreute. Nicht zuletzt aus ökologischen Gründen verzichten wir zukünftig auf eine CD-Rom. Stattdessen kann das Quantum Verzeichnis in digitaler Form über unseren Webshop (www.buchkurier.de) kostenlos heruntergeladen werden. Die Datei darf gerne kopiert und weitergegeben werden. -

Historic Environment Action Plan West Wight Chalk Downland
Directorate of Community Services Director Sarah Mitchell Historic Environment Action Plan West Wight Chalk Downland Isle of Wight County Archaeology and Historic Environment Service October 2008 01983 823810 archaeology @iow.gov.uk Iwight.com HEAP for West Wight Chalk Downland. INTRODUCTION The West Wight Chalk Downland HEAP Area has been defined on the basis of geology, topography and historic landscape character. It forms the western half of a central chalk ridge that crosses the Isle of Wight, the eastern half having been defined as the East Wight Chalk Ridge . Another block of Chalk and Upper Greensand in the south of the Isle of Wight has been defined as the South Wight Downland . Obviously there are many similarities between these three HEAP Areas. However, each of the Areas occupies a particular geographical location and has a distinctive historic landscape character. This document identifies essential characteristics of the West Wight Chalk Downland . These include the large extent of unimproved chalk grassland, great time-depth, many archaeological features and historic settlement in the Bowcombe Valley. The Area is valued for its open access, its landscape and wide views and as a tranquil recreational area. Most of the land at the western end of this Area, from the Needles to Mottistone Down, is open access land belonging to the National Trust. Significant historic landscape features within this Area are identified within this document. The condition of these features and forces for change in the landscape are considered. Management issues are discussed and actions particularly relevant to this Area are identified from those listed in the Isle of Wight HEAP Aims, Objectives and Actions. -

Neolithic & Early Bronze Age Isle of Wight
Neolithic to Early Bronze Age Resource Assessment The Isle of Wight Ruth Waller, Isle of Wight County Archaeology and Historic Environment Service September 2006 Inheritance: The map of Mesolithic finds on the Isle of Wight shows concentrations of activity in the major river valleys as well two clusters on the north coast around the Newtown Estuary and Wooton to Quarr beaches. Although the latter is likely due to the results of a long term research project, it nevertheless shows an interaction with the river valleys and coastal areas best suited for occupation in the Mesolithic period. In the last synthesis of Neolithic evidence (Basford 1980), it was claimed that Neolithic activity appears to follow the same pattern along the three major rivers with the Western Yar activity centred in an area around the chalk gap, flint scatters along the River Medina and greensand activity along the Eastern Yar. The map of Neolithic activity today shows a much more widely dispersed pattern with clear concentrations around the river valleys, but with clusters of activity around the mouths of the four northern estuaries and along the south coast. As most of the Bronze Age remains recorded on the SMR are not securely dated, it has been difficult to divide the Early from the Late Bronze Age remains. All burial barrows and findspots have been included within this period assessment rather than the Later Bronze Age assessment. Nature of the evidence base: 235 Neolithic records on the County SMR with 202 of these being artefacts, including 77 flint or stone polished axes and four sites at which pottery has been recovered. -

Get Fit the One Card
The community magazine for the Isle of Wight Issue 29 September 2010 Get fit the One Card way See inside for new Fall into Fitness campaign WELCOME The community magazine for the Isle of Wight How to contact us Issue 29 September 2010 Welcome to September’s One Island If you have community news to share In this edition we feature a on Ryde fire station, currently with other readers, we would like to hear special eight-page annual at the forefront of the from you. We also welcome your letters. report supplement, giving important modernisation You can contact us by post, email or you details of the council’s plans for the Island’s fire and telephone. achievements on your rescue service, and take a look behalf in 2009/2010 and at the exciting Fall into Fitness Post One Island, Communications, the priorities as we move initiative at the Island’s leisure County Hall, Newport PO30 1UD through the next financial centres – including special Email [email protected] year and beyond. offers available through the Get fit the We also turn the spotlight One Card scheme. Telephone 823105 One Card w a y See inside for new Fall into Fitness campaign USEFUL CONTACTS Advertising in EMERGENCY NUMBERS COUNCIL MEETINGS Isle of Wight Council, County In an emergency dial 999 Unless otherwise stated, One Island all meetings are in public Hall, Newport PO30 1UD Fire and rescue control More than 43,000 copies of Fax: 823333 centre (24hrs) 525121 at County Hall. Call 823200 24 hours before a meeting to One Island are distributed Email: Out of hours: ensure it is going ahead and throughout the Island every [email protected] Highways 525121 to check if any items are likely two months. -

Isle of Wight Rivers
KENT AREA HAMPSHIRE Maidstone AREA Winchester Worthing SUSSEX AREA Area Administrative Boundaries Regional Boundary Area Office Rivers of Regional Headquarters the Isle ENVIRONMENT AGENCY GENERAL ENQUIRY LINE of Wight 0845 933 3111 ENVIRONMENT AGENCY FLOODLINE 0845 988 1188 ENVIRONMENT AGENCY EMERGENCY HOTLINE 0800 80 70 60 FACT FILES 9 Rivers of the Isle of Wight Environment Agency - a better organisation works for the public and environment in England and Wales has specific duties and powers. Lymington Cowes R i East v e The Solent r Cowes for present and future generations. M e The Solent d i n Nationally, around 15 million hectares a Fishbourne ek re Milford- Wootton C Northwood n The Environment Agency is one of the of land are managed by the Agency k o Newtown River t on-Sea o t o o r o B s W ' r world's most powerful environmental along with 36,000km of rivers and e Nettlestone n R e m o l d B v a a g l H e B P a ck Ryde Yarmouth rn ro b e o rid t k g watchdogs, regulating air, land and 5,000km of coastline, including more es e W Bro o Th k St Helens Bembridge o rl water. As 'guardians of the than 2 million hectares of coastal ey Br o Yar o e k n rn r u environment' the Agency has legal waters. Totland ste Bo Newport e l r u a W Y Ca Brading n er st duties to protect and improve the Freshwater Ea There are eight regional offices, which R i r a v Y environment throughout England and e r n r M e t are split into 26 area offices. -

Location Address1 Address2 Address3 Postcode Asset Type
Location Address1 Address2 Address3 Postcode Asset Type Description Tenure Alverstone Land Alverstone Shute Alverstone PO36 0NT Land Freehold Alverstone Grazing Land Alverstone Shute Alverstone PO36 0NT Grazing Land Freehold Arreton Branstone Farm Study Centre Main Road Branstone PO36 0LT Education Other/Childrens Services Freehold Arreton Stockmans House Main Road Branstone PO36 0LT Housing Freehold Arreton St George`s CE Primary School Main Road Arreton PO30 3AD Schools Freehold Arreton Land Off Hazley Combe Arreton PO30 3AD Non-Operational Freehold Arreton Land Main Road Arreton PO30 3AB Schools Leased Arreton Land Arreton Down Arreton PO30 2PA Non-Operational Leased Bembridge Bembridge Library Church Road Bembridge PO35 5NA Libraries Freehold Bembridge Coastguard Lookout Beachfield Road Bembridge PO35 5TN Non-Operational Freehold Bembridge Forelands Middle School Walls Road Bembridge PO35 5RH Schools Freehold Bembridge Bembridge Fire Station Walls Road Bembridge PO35 5RH Fire & Rescue Freehold Bembridge Bembridge CE Primary Steyne Road Bembridge PO35 5UH Schools Freehold Bembridge Toilets Lane End Bembridge PO35 5TB Public Conveniences Freehold Bembridge RNLI Life Boat Station Lane End Bembridge PO35 5TB Coastal Freehold Bembridge Car Park Lane End Forelands PO35 5UE Car Parks Freehold Bembridge Toilets Beach Road / Station Road Bembridge PO35 5NQ Public Conveniences Freehold Bembridge Toilet High Street Bembridge PO35 5SE Public Conveniences Freehold Bembridge Toilets High Street Bembridge PO35 5SD Public Conveniences Freehold Bembridge -

BULLETIN Aug 2008
August 2008 Issue no. 50 Bulletin Established 1919 www.iwnhas.org Contents Page(s) Page(s) President`s Address 1 In Praise of Ivy 8-11 Notice Board 2 Note from 1923 Proceedings 11 Country Notes 3-4 General Meetings 12-20 Undercliff Walls Survey 4-5 Section Meetings 20-30 Society Library 5 Membership Secretaries` Notes 31 Andy`s Notes 5-6 Megalith Monuments 6-8 An Unusual Moth 8 President's Address On a recent visit to the Society's Headquarters at Ventnor I was handed a piece of paper outlin- ing the duties of the President. One clause seemed directly written to me - the President was “not ex- pected to be expert in all the fields covered by the various sections." As most of you will know, unlike past Presidents who have been specialists, I am very much an ordinary member with a general interest in natural history and archaeology, but my membership over many years has widened my knowledge and kept up a spirit of inquiry. With this in mind last May I joined the general meeting exploring the ground around the glass- houses of Wight Salads, somewhere completely new to me. It proved to be two hours of continuous in- terest as we wandered by the new lakes created from the water used in the hydroponic growing system. With experts in identifying grasses, mosses, flowers, birds all to hand, and all more than willing to point out, identify and explain when I asked any question. It was a real illustration of teaching and learning in the best way. -

Cattle Bulls, Baiting & Hard Cheese
f olkonwight Island Folk History Adapted from Cock & Bull Stories: Animals in Isle of Wight Folklore, Dialect and Cultural History (2008), by Alan R Phillips C ATTLE BULLS , BAITING & HARD CHEESE Numerous ox bones, many of which had been split open for the extraction of marrow, together with the teeth bones of a horse, were discovered in 1936 by Hubert Poole in a Mesolithic deposit on the east bank of the Newtown River. By the Iron Age dairy and b eef cattle together with sheep would have grazed the Island's meadows and oxen would have have been yoked to a wooden plough, or 'ard'. Courtesy of Isle of Wight Heritage Service Regarding the three - branched prehistoric flint implement known as a tribra ch, which has remained something of a mystery since its discovery most probably at Ventnor in the 1850s, Hubert Poole conjectured in 1941 that if displayed with its longer arm pointing downwards it bears a rough resemblance to the horned head of a bull, wh ich could conceivably have been mounted on a staff and carried in procession. This remains arguably still the best interpretation, though whether one would wish to concur with Poole that it might have been part of a phallic cult (all the rage in archaeolog ical circles when Poole was writing) is perhaps less likely. Poole went on to draw an analogy with the Royal Antediluvian Order of Buffaloes on the Island, which used to carry at an annual church parade a pair of bull's horns mounted on a staff in much th e same way that others carried a banner. -

Multi-Agency Flood Response Plan
NOT PROTECTIVELY MARKED Multi-Agency Flood Response Plan ANNEX 4 TECHNICAL INFORMATION Prepared By: Isle of Wight Local Authority Emergency Management Version: 1.1 Island Resilience Forum 245 Version 1.0 Multi-Agency Flood Response Plan Date: March 2011 May 2010 BLANK ____________________________________________________________________________________________ Island Resilience Forum 246 Version 1.1 Multi-Agency Flood Response Plan March 2011 Not Protectively Marked Annex 4 – Technical Information Contents ____________________________________________________________________________________________ Annex 4 – Technical Information Page Number 245 Section 1 – Weather Forecasting and Warning • Met Office 249 • Public Weather Service (PWS) 249 • National Severe Weather Warning Service (NSWWS) 250 • Recipients of Met Office Weather Warnings 255 • Met Office Storm Tide Surge Forecasting Service 255 • Environment Monitoring & Response Centre (EMARC) 256 • Hazard Manager 256 Section 2 – Flood Forecasting • Flood Forecasting Centre 257 • Flood Forecasting Centre Warnings 257 • Recipients of Flood Forecasting Centre Warnings 263 Section 3 – Flood Warning • Environment Agency 265 • Environment Agency Warnings 266 • Recipients of Environment Agency Flood Warnings 269 Section 4 – Standard Terms and Definitions • Sources/Types of Flooding 271 • Affects of Flooding 272 • Tide 273 • Wind 276 • Waves 277 • Sea Defences 279 • Forecasting 280 Section 5 – Flood Risk Information Maps • Properties at Flood Risk 281 • Areas Susceptible to Surface Water Flooding -

Location Address 1 Address 2 Location Postcode Asset Type
Location Address 1 Address 2 Location Postcode Asset Type Description Site Description Code Tenure Alverstone Grazing Land Alverstone Shute Alverstone PO36 0NT Grazing Land Alverstone Land at Alverstone Shute 02084 Freehold Appley Appley Beach Huts Esplanade Ryde PO33 1ND Esplanade, Parks & Gardens Ryde Appley Beach Huts 03190 Freehold Appley Beach area The Esplanade Ryde PO33 1ND Esplanade, Parks & Gardens Ryde Part of Beach at Appley M214 03267 Freehold Arreton Branstone Farm Study Centre Main Road Arreton PO36 0LT Education Non-Schools(Youth Centre, Residence etc) Arreton Branstone Farm Study Centre 00045 Freehold Arreton Stockmans House Main Road Arreton PO36 0LT Housing Arreton Stockmans House 00251 Freehold Arreton St George`s CE Primary School Main Road Arreton PO30 3AD Schools Arreton St George`s CE Primary School 00733 Freehold Arreton Land Off Hazley Combe Arreton PO30 3AD Non-Operational Arreton Land Adjacent Sewerage Works off Hazley Combe 01176 Freehold Arreton Land Garlic Festival Site Main Road Arreton PO36 0LT Non-Operational Arreton Land at Bathingbourne Site (Garlic Festival Site) 01821 Freehold Arreton Land Main Road Arreton PO30 3AB Schools Arreton Extra Playing Field Land adjacent School X0276 Leasehold Arreton Land Arreton Down Arreton PO30 2PA Non-Operational Arreton Land at Michael Moreys Hump X0277 Leasehold Bembridge Bembridge Library Church Road Bembridge PO35 5NA Libraries Bembridge Community Library 00028 Freehold Bembridge Coastguard Lookout Beachfield Road Bembridge PO35 5TN Non-Operational Bembridge Coastguard -

The Isle of Wight in the English Landscape
THE ISLE OF WIGHT IN THE ENGLISH LANDSCAPE: MEDIEVAL AND POST-MEDIEVAL RURAL SETTLEMENT AND LAND USE ON THE ISLE OF WIGHT HELEN VICTORIA BASFORD A study in two volumes Volume 1: Text and References Thesis submitted in partial fulfilment of the requirements of Bournemouth University for the degree of Doctor of Philosophy January 2013 2 Copyright Statement This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with its author and due acknowledgement must always be made of the use of any material contained in, or derived from, this thesis. 3 4 Helen Victoria Basford The Isle of Wight in the English Landscape: Medieval and Post-Medieval Rural Settlement and Land Use Abstract The thesis is a local-scale study which aims to place the Isle of Wight in the English landscape. It examines the much discussed but problematic concept of ‘islandness’, identifying distinctive insular characteristics and determining their significance but also investigating internal landscape diversity. This is the first detailed academic study of Isle of Wight land use and settlement from the early medieval period to the nineteenth century and is fully referenced to national frameworks. The thesis utilises documentary, cartographic and archaeological evidence. It employs the techniques of historic landscape characterisation (HLC), using synoptic maps created by the author and others as tools of graphic analysis. An analysis of the Isle of Wight’s physical character and cultural roots is followed by an investigation of problems and questions associated with models of settlement and land use at various scales.