RSC Advances
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Regulation of Plant Monoterpene Biosynthesis in Relation to Fragrance
Molecular Regulation of Plant Monoterpene Biosynthesis In Relation To Fragrance Mazen K. El Tamer Promotor: Prof. Dr. A.G.J Voragen, hoogleraar in de Levensmiddelenchemie, Wageningen Universiteit Co-promotoren: Dr. ir. H.J Bouwmeester, senior onderzoeker, Business Unit Celcybernetica, Plant Research International Dr. ir. J.P Roozen, departement Agrotechnologie en Voedingswetenschappen, Wageningen Universiteit Promotiecommissie: Dr. M.C.R Franssen, Wageningen Universiteit Prof. Dr. J.H.A Kroeze, Wageningen Universiteit Prof. Dr. A.J van Tunen, Swammerdam Institute for Life Sciences, Universiteit van Amsterdam. Prof. Dr. R.G.F Visser, Wageningen Universiteit Mazen K. El Tamer Molecular Regulation Of Plant Monoterpene Biosynthesis In Relation To Fragrance Proefschrift ter verkrijging van de graad van doctor op gezag van de rector magnificus van Wageningen Universiteit, Prof. dr. ir. L. Speelman, in het openbaar te verdedigen op woensdag 27 november 2002 des namiddags te vier uur in de Aula Mazen K. El Tamer Molecular Regulation Of Plant Monoterpene Biosynthesis In Relation To Fragrance Proefschrift Wageningen Universiteit ISBN 90-5808-752-2 Cover and Invitation Design: Zeina K. El Tamer This thesis is dedicated to my Family & Friends Contents Abbreviations Chapter 1 General introduction and scope of the thesis 1 Chapter 2 Monoterpene biosynthesis in lemon (Citrus limon) cDNA isolation 21 and functional analysis of four monoterpene synthases Chapter 3 Domain swapping of Citrus limon monoterpene synthases: Impact 57 on enzymatic activity and -

Review Article a Review on Anti-Oxidative Herbs PL
INTERNATIONAL JOURNAL OF PHARMACEUTICAL AND CHEMICAL SCIENCES ISSN: 22775005 Review Article A Review on Anti-oxidative Herbs PL. Rajagopal1*, VB. Narayana Swamy2, SS. Kiron3 and KR. Sreejith4 1Department of Pharmacognosy, Academy of Pharmaceutical Sciences, Pariyaram Medical College, Kannur, Kerala, South India. 2Professor and Principal, Department of Pharmacognosy, Karaveli College of Pharmacy, Mangalore, Karnataka, South India. 3Department of Pharmacy Practice, Academy of Pharmaceutical Sciences, Pariyaram Medical College, Kannur, Kerala, South India. 4Department of Pharmaceutical Chemistry, Academy of Pharmaceutical Sciences, Pariyaram Medical College, Kannur, Kerala, South India. ABSTRACT Plants are valuable source of the therapeutic agents in the armory of modern medicine. The method of drug development from plant sources is based on a sequence of operation leading mainly toward the isolation of pure natural products. An antioxidant is a molecule that inhibits the oxidation of other molecules. Antioxidants have been investigated for the prevention of diseases such as cancer, coronary heart disease and even altitude sickness. The major sources of anti-oxidants are reported to be from the natural source, especially from plant source. Key words: Anti oxidant, Free radical, Medicinal Plants INTRODUCTION pharmacological studies to ascertain their Till date as such no set definition of the term therapeutic properties. (Bakru H.K. 1992). antioxidant exists. Scientists are still striving In this review an attempt has been made o hard to find out the role of particular dietary compile most of the Natural Herbs which supplements in body that have potent health possess ant oxidative property. Following are benefits. Since, different antioxidant the list of such medicinal herbs which are compounds found in diet considerably vary reported to be an antioxidant. -

Leaf and Flower Volatile Oil Components of Two Thyme Taxa Origanum Onites L
Avrupa Bilim ve Teknoloji Dergisi European Journal of Science and Technology Sayı 17, S. 346-350, Aralık 2019 No. 17, pp. 346-350, December 2019 © Telif hakkı EJOSAT’a aittir Copyright © 2019 EJOSAT Araştırma Makalesi www.ejosat.com ISSN:2148-2683 Research Article Leaf and Flower Volatile Oil Components of Two Thyme Taxa Origanum onites L. and Thymbra spicata var. spicata L. in Turkey Ayşe Gül Sarıkaya1* 1 Bursa Technical University, Faculty of Forestry, Bursa-Turkey (ORCID: 0000-0002-0641-4445) (İlk Geliş Tarihi 10 Eylül 2019 ve Kabul Tarihi 14 Ekim 2019) (DOI: 10.31590/ejosat.618187) ATIF/REFERENCE: Sarıkaya, A. (2019). Leaf and Flower Volatile Oil Components of Two Thyme Taxa Origanum onites L. and Thymbra spicata var. spicata L. in Turkey. Avrupa Bilim ve Teknoloji Dergisi, (17), 346-350. Abstract Medicinal and aromatic plants have a special importance with volatile oil components. Lamiaceae family members are important in pharmacology and perfumery industry because they contain volatile and aromatic oil. Origanum onites L. and Thymbra spicata var. spicata L. are the most widely used and most exported species. The volatile components of the leaves and flowers of Origanum onites L. and Thymbra spicata var. spicata taxa were determined by Headspace Solid Phase Microextraction (HS-SPME) technique combined with gas chromatography/mass spectrometry (GC / MS). 33 different components of Origanum onites were identified and the main components were p-cymene (11.45%), γ-terpinene (11.89%), linalool (14.35%), thymol (20.03%) and carvacrol (26.91%), respectively. For Thymbra spicata var. spicata L., 36 different compounds were identified and the main components were p-cymene (11.72%), γ-terpinene (10.96%), linalool (13.44%), thymol (18.92%) and carvacrol (27.34%), respectively. -

Camphene, A3-Carene, Limonene, and Ot=Terpinene
Environ. Sci. Technol. 1999, 33,4029-4033 The hydrocarbon emissions are typically divided into two Thermal Degradation of Terpenes: categories: (a) the condensed hydrocarbons of higher molecular weight that are responsible in part for the blue- Camphene, A3-Carene, Limonene, haze plume characteristic of dryer emissions and (b) the and ot=Terpinene lower molecular weight hydrocarbons (C+&), generally referred to as volatile hydrocarbons. Both nongaseous (condensed) and gaseous (volatile) hydrocarbons emitted GERALD W. MCGRAW,*,+ by wood dryers have been analyzed by a number of workers RICHARD W. HEMINGWAY,* LEONARD L. INGRAM, JR.,s (2-s). In general, the nongaseous fraction consists of a CATHERINE S. CANADY,’ AND mixture of resin acids and fatty acids and their esters as well WILLIAM B. MCGRAW+ as some sesquiterpenoid compounds and undefined oxida- tion products. The gaseous fraction is primarily made up of of Chemistry, Louisiana College, monoterpenes present in the wood and some of their Pineuille, Louisiana 71359, Southern Research Station, USDA Forest Service, 2500 Shreveport Highway, oxidation products. Comparatively little is known about the Pineuille, Louisiana 71360, and Forest Products Lab, yields, structures, and biological properties of oxidation Department of Forest Products, Mississippi State University, products of monoterpenes. Mississippi State, Mississippi 39762-9820 Cronn et al. (3) studied the gaseous emissions from a number of veneer dryers at mills in the northwest and southern U.S. From a plywood veneer dryer in the southern U.S. using a mixture of loblolly and shortleaf pines, it was Emissions from wood dryers have been of some concern found that terpenes accounted for 98.9% of the total gaseous for a number of years, and recent policy changes by the hydrocarbon emissions. -

A Genomic Approach to Characterization of the Citrus Terpene Synthase Gene Family
Genetics and Molecular Biology, 30, 3 (suppl), 832-840 (2007) Copyright by the Brazilian Society of Genetics. Printed in Brazil www.sbg.org.br Research Article A genomic approach to characterization of the Citrus terpene synthase gene family Marcelo Carnier Dornelas and Paulo Mazzafera Departamento de Fisiologia Vegetal, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, SP, Brazil. Abstract Terpenes are a very large and structurally diverse group of secondary metabolites which are abundant in many es- sential oils, resins and floral scents. Additionally, some terpenes have roles as phytoalexins in plant-pathogen rela- tionships, allelopathic inhibitors in plant-plant interactions, or as airborne molecules of plant-herbivore multitrophic signaling. Thus the elucidation of the biochemistry and molecular genetics of terpenoid biosynthesis has paramount importance in any crop species. With this aim, we searched the CitEST database for clusters of expressed sequence tags (ESTs) coding for terpene synthases. Herein is a report on the identification and in silico characterization of 49 putative members of the terpene synthase family in diverse Citrus species. The expression patterns and the possible physiological roles of the identified sequences are also discussed. Key words: secondary metabolism, flavor, aroma, plant protection, terpene synthesis. Received: July 21, 2006; Accepted: April 2, 2007. Introduction respectively. Polyterpenes are all terpenes containing more Terpenes are found widely distributed in the plant than eight isoprene units, which include all natural rubbers kingdom, from lichens and algae to higher plants. Although (Goodwin, 1967; Croteau et al., 2000). terpenes cover a wide range of compounds with diverse Elucidation of the biochemistry and molecular genet- structure, the word terpene has been frequently associated ics of terpenoid biosynthesis has made rapid progress in re- with essential oils, which are volatile compounds belong- cent years (Rohdich et al., 2005). -

Monoterpenes in the Glandular Trichomes of Tomato Are Synthesized
Monoterpenes in the glandular trichomes of tomato SEE COMMENTARY are synthesized from a neryl diphosphate precursor rather than geranyl diphosphate Anthony L. Schilmillera,1, Ines Schauvinholdb,1, Matthew Larsonc, Richard Xub, Amanda L. Charbonneaua, Adam Schmidtb, Curtis Wilkersona,d, Robert L. Lasta,d, and Eran Picherskyb,2 aDepartments of Biochemistry and Molecular Biology and dPlant Biology, and cBioinformatics Core, Research Technology Support Facility, Michigan State University, East Lansing, MI 48824-1319; and bDepartment of Molecular, Cellular, and Developmental Biology, University of Michigan, Ann Arbor, MI 48109-1048 Communicated by Anthony R. Cashmore, University of Pennsylvania, Philadelphia, PA, April 20, 2009 (received for review December 19, 2008) We identified a cis-prenyltransferase gene, neryl diphosphate (FPP), and diterpene synthases use the C20-diphosphate inter- synthase 1 (NDPS1), that is expressed in cultivated tomato (Sola- mediate E,E,E-geranylgeranyl diphosphate (GGPP) (7). The num lycopersicum) cultivar M82 type VI glandular trichomes and enzymes that synthesize these intermediates are designated as encodes an enzyme that catalyzes the formation of neryl diphos- trans-prenyltransferases, and they consist of a family of struc- phate from isopentenyl diphosphate and dimethylallyl diphos- turally related proteins with representatives found in all branches phate. mRNA for a terpene synthase gene, phellandrene synthase of life (8). 1 (PHS1), was also identified in these glands. It encodes an enzyme In contrast, the isoprene units of some long-chain plant that uses neryl diphosphate to produce -phellandrene as the terpenoids such as rubber and dolichols (the latter group of major product as well as a variety of other monoterpenes. The compounds is present in bacteria, fungi, and animals as well) are profile of monoterpenes produced by PHS1 is identical with linked to each other in the cis (Z) conformation (9, 10). -

Effect of Nanoencapsulation on Volatile Constituents, And
www.nature.com/scientificreports OPEN Efect of nanoencapsulation on volatile constituents, and antioxidant and anticancer activities of Algerian Origanum glandulosum Desf. essential oil Hatem Ali1,2, Abdel Rahman Al-Khalifa1, Abdelhakim Aouf3, Habiba Boukhebti4 & Amr Farouk5* Nanoencapsulation is an attractive novel technique used for incorporating essential oils in food preparations and pharmaceutical formulae. This study investigated the efect of nanoencapsulation on the composition of volatile compounds, as well as the antioxidant and anticancer activities of hydrodistilled (HD) Origanum glandulosum Desf. Oil, which was encapsulated into nanocapsules via High Speed Homogenization (HSH) and into nanoemulsions through High Pressure Homogenization (HPH). Thirty-two volatile components were identifed using Gas Chromatography-Mass Spectrometry analysis (GC-MS) in HD essential oil representing 99.04% of the total oil content. GC-MS analysis showed that the use of HPH to prepare nanoemulsions negatively afected the active compounds present in HD oil, particularly carvacrol and thymol, whereas the use of HSH led to signifcant quantitative diferences in the composition of volatiles between HD oil and nanocapsules but generated the same profle. Consistent with the diferences in total phenolics, total favonoids, and volatiles identifed in HD and nanoparticles, HD essential oil exhibited a higher antioxidant activity (IC50 4.22 mg/ mL) than nanocapsules (IC50 57.51 mg/mL) and nanoemulsion (IC50 78.50 mg/mL), while nanocapsules showed the strongest cytotoxic efect on liver cancer cell line Hep-G2 (54.93 μg/mL) in comparison to HD oil (73.13 μg/mL) and nanoemulsions (131.6 μg/mL). Te Origanum genus (Lamiaceae family) includes approximately 38 species that have been studied extensively for potential importance, and uses in favoring foods and traditional medicine due to their pharmacological charac- teristics1. -
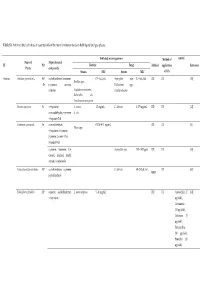
Table S1. Antimicrobial Activities of Essential Oils of the Main Functional Foods in Both Liquid and Gas Phases
Table S1. Antimicrobial activities of essential oils of the main functional foods in both liquid and gas phases Inhibited microorganisms Method of control Name of Major chemical PF PO Bacteria Fungi Method application Reference Plants compounds Strains MIC Strains MIC of EOs Apiaceae Anethum graveolens L. AP, α-phellandrene, limonene, 0.5‒5μL/mL Aspergillus nige, 0.5‒5μL/mL DD DC [58] Bacillus spp., Fr p-cymene, carvone, Trichoderma spp., dillether Staphylococcus aureus, Candida albicans Escherichia coli, Pseudomonas aerogenosa Bunium persicum Fr γ-terpinene, S. aureus, 1.5 mg/mL C. albicans 0.375 mg/mL DD DC [12] cuminaldehyde, p-cymene, E. coli γ-terpinen-7-al Cuminum cyminum L. Se cuminlaldehyde, 0.078–0.31 mg/mL DD DC [8] Vibrio spp. γ-terpinene, o-cymene, β-pinene, 2-caren-10-al, 3-caren-10-al α-pinene, limonene, 1,8- Aspergillus spp. 750‒1000 ppm DD DC [59] cineole, linalool, linalyl acetate, α-terpineole Echinophora Cinerea Boiss AP α-phellandrene, α-pinene, C. albicans 60‒250 μL/mL DC [60] MBD β-phellandrene Echinophora platyloba AP ocimene, α-phellandrene, L. monocytogenes 5‒10 mg/mL DD DC Amoxicillin 25 [61] γ-terpinene μg/disk), Gentamicin (10 μg/disk), Cefexime (5 μg/disk), Tetracycline (30 μg/disk), Penicillin (10 μg/disk) ocimene, 2,3-dimethyl-1,3- 0.63‒0.31 DD DC [62] A. flavus, Penicilium cyclohexadiene, α–pinene, mg./mL γ-dodecalactone expansum, Fusarium graminearum Foeniculum vulgare Mill. Se Shigella dysenteriae 0.125 mg/mL Oc DC [48] trans-anethole, estragole, limonene, fenchone estragole, limonene, 0.16–0.2 DD DC [63] C. -

A Study on the Biosynthesis of Camphor
A STUDY ON THE BIOSYNTHESIS OF CAMPHOR By THERESE MARY ATLAY B.Sc.(Hons.) University of Exeter, 1976. A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE in the Department of Chemistry. We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA May 1983. © Therese Mary Atlay, 1983. In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the head of my department or by his or her representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Department of OH Q^NA i The University of British Columbia 1956 Main Mall Vancouver, Canada V6T 1Y3 Date Starvd r^Sib DE-6 (3/81) - ii - ABSTRACT. This thesis describes an investigation of the biosynthesis of camphor. Camphor, a bicyclic monoterpene, has been shown to be bio- synthesised from.geranyl pyrophosphate or its isomers (neryl pyro• phosphate or linaloyl pyrophosphate) and several mechanisms have been proposed for this cyclisation process. geranyl pyrophosphate camphor In an attempt to differentiate the origin of the C(8) and C(9) methyl groups,, feeding experiments with appropriately labelled pre- cursors were attempted. The two precursors used were [2- H^J-mevalonic r 2 i acid and [8- H^J-linalool. -

Green Scientific Labs, LLC
CERTIFICATE OF ANALYSIS ISO/IEC 17025:2017 ACCREDITATION #103104 Order #: 42542 Oliver’s Harvest Order Name: Oliver's Harvest 3361 Fairlane Farms Road, Suite 2B Pain Relieving Cream with Wellington FL, 33414 Capsaicin 2 oz (866) 634-3134 Batch#: DO202CI300 [email protected] Received: 11/27/2019 Completed: 12/03/2019 Sample Cannabinoids Test SHIMADZU INTEGRATED UPLC-PDA GSL SOP 400 PREPARED: 11/27/2019 16:19:31 UPLOADED: 11/29/2019 14:20:20 Cannabinoids LOQ weight(%) mg/g mg/unit D9-THC 10 PPM 0.019% 0.187 10.6 THCA 10 PPM N/D N/D N/D CBD 10 PPM 0.670% 6.696 379.7 CBDA 20 PPM 0.006% 0.060 3.4 CBDV 20 PPM N/D N/D N/D CBC 10 PPM 0.024% 0.243 13.8 CBN 10 PPM N/D N/D N/D CBG 10 PPM 0.010% 0.095 5.4 0.019% 0.675% CBGA 20 PPM N/D N/D N/D D9-THC Total CBD D8-THC 10 PPM N/D N/D N/D THCV 10 PPM N/D N/D N/D TOTAL D9-THC 0.019% 0.019% 10.6 TOTAL CBD* 0.675% 6.749 382.7 412.9 mg 382.7 mg TOTAL CANNABINOIDS 0.729% 7.281 412.9 Cannabinoids per CBD per unit unit 1 unit = 56.699 grams per unit x Cannabinoid concentration Reporting Limit 10 ppm *Total CBD = CBD + CBDA x 0.877 N/D - Not Detected, B/LOQ - Below Limit of Quantification 4001 SW 47th Avenue Suite 208 Davie, FL 33314 1-833-TEST-CBD [email protected] Green Scientific Labs uses its best efforts to deliver high quality results and to verify that the data contained therein are based on sound scientific judgment and levels listed are guidelines only and all data was reported based on standard laboratory procedures and deviations. -

Biosynthesis of the Phenolic Monoterpenes, Thymol and Carvacrol, by Terpene Synthases and Cytochrome P450s in Oregano and Thyme
Biosynthesis of the phenolic monoterpenes, thymol and carvacrol, by terpene synthases and cytochrome P450s in oregano and thyme Dissertation Zur Erlangung des akademischen Grades doctor rerum naturalium (Dr. rer. nat.) vorgelegt dem Rat der Biologisch-Pharmazeutischen Fakultät der Friedrich-Schiller-Universität Jena von Diplom-Biologe Christoph Crocoll geboren am 11. Februar 1977 in Kassel Gutachter: 1. Prof. Dr. Jonathan Gershenzon, Max-Planck-Institut für chemische Ökologie, Jena 2. Prof. Dr. Christian Hertweck, Hans-Knöll-Institut, Jena 3. Prof. Dr. Harro Bouwmeester, Wageningen University, Wageningen Tag der öffentlichen Verteidigung: 11.02.2011 Biosynthesis of the phenolic monoterpenes, thymol and carvacrol, by terpene synthases and cytochrome P450s in oregano and thyme Christoph Crocoll - Max-Planck-Institut für chemische Ökologie - 2010 Contents 1 General introduction ................................................................................................. 1 2 Chapter I ................................................................................................................... 13 Terpene synthases of oregano (Origanum vulgare L.) and their roles in the pathway and regulation of terpene biosynthesis 2.1 Abstract ............................................................................................................................ 13 2.2 Introduction ...................................................................................................................... 14 2.3 Materials and Methods .................................................................................................... -
Exp 3 Isolation and Analysis Name ______Of Citrus Oils Due Date for Report in Syllabus
CHEM 8L, Binder UCSC Experiment 3: ISOLATION AND ANALYSIS OF CITRUS OILS A GREEN-CHEMISTRY APPROACH Reading Assignment Mohrig Chapters 12 & 20 (Distillation and Gas Chromatography) In this experiment students will isolate the essential oil of one of the following citrus fruits: lemon, lime, grapefruit, or orange. Steam distillation will be used to obtain the oil from citrus peels. Essential oils are mixtures of volatile compounds made by plants to communicate with their environments. They are used to attract insects and other animals to help in the fertilization and propagation processes. Some are also herbicides used by plants to defend their territory from aggressive vegetation. These mixtures are called oils because they are immiscible with water and, being lighter than water, separate as the upper layer. By mixing the freshly grated peels of the citrus fruit of your choice with water and carrying out a distillation you will obtain a distillate that consists largely of water and small amounts of citrus oil. The distillate may look cloudy because of the emulsification of the oil in water, but if left to settle, it will separate into two layers: a large aqueous bottom layer and a small oily upper layer. The separation of the oily upper layer from the bottom aqueous layer can be difficult, especially if the volume of oil is small. To circumvent this problem, the oil is usually separated from the water by liquid-liquid extraction with an organic solvent. This whole process is very effective in removing the oil from the water but requires the use of organic solvents, which are expensive and must be handled with special precautions.