Camphene, A3-Carene, Limonene, and Ot=Terpinene
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Regulation of Plant Monoterpene Biosynthesis in Relation to Fragrance
Molecular Regulation of Plant Monoterpene Biosynthesis In Relation To Fragrance Mazen K. El Tamer Promotor: Prof. Dr. A.G.J Voragen, hoogleraar in de Levensmiddelenchemie, Wageningen Universiteit Co-promotoren: Dr. ir. H.J Bouwmeester, senior onderzoeker, Business Unit Celcybernetica, Plant Research International Dr. ir. J.P Roozen, departement Agrotechnologie en Voedingswetenschappen, Wageningen Universiteit Promotiecommissie: Dr. M.C.R Franssen, Wageningen Universiteit Prof. Dr. J.H.A Kroeze, Wageningen Universiteit Prof. Dr. A.J van Tunen, Swammerdam Institute for Life Sciences, Universiteit van Amsterdam. Prof. Dr. R.G.F Visser, Wageningen Universiteit Mazen K. El Tamer Molecular Regulation Of Plant Monoterpene Biosynthesis In Relation To Fragrance Proefschrift ter verkrijging van de graad van doctor op gezag van de rector magnificus van Wageningen Universiteit, Prof. dr. ir. L. Speelman, in het openbaar te verdedigen op woensdag 27 november 2002 des namiddags te vier uur in de Aula Mazen K. El Tamer Molecular Regulation Of Plant Monoterpene Biosynthesis In Relation To Fragrance Proefschrift Wageningen Universiteit ISBN 90-5808-752-2 Cover and Invitation Design: Zeina K. El Tamer This thesis is dedicated to my Family & Friends Contents Abbreviations Chapter 1 General introduction and scope of the thesis 1 Chapter 2 Monoterpene biosynthesis in lemon (Citrus limon) cDNA isolation 21 and functional analysis of four monoterpene synthases Chapter 3 Domain swapping of Citrus limon monoterpene synthases: Impact 57 on enzymatic activity and -

Review Article a Review on Anti-Oxidative Herbs PL
INTERNATIONAL JOURNAL OF PHARMACEUTICAL AND CHEMICAL SCIENCES ISSN: 22775005 Review Article A Review on Anti-oxidative Herbs PL. Rajagopal1*, VB. Narayana Swamy2, SS. Kiron3 and KR. Sreejith4 1Department of Pharmacognosy, Academy of Pharmaceutical Sciences, Pariyaram Medical College, Kannur, Kerala, South India. 2Professor and Principal, Department of Pharmacognosy, Karaveli College of Pharmacy, Mangalore, Karnataka, South India. 3Department of Pharmacy Practice, Academy of Pharmaceutical Sciences, Pariyaram Medical College, Kannur, Kerala, South India. 4Department of Pharmaceutical Chemistry, Academy of Pharmaceutical Sciences, Pariyaram Medical College, Kannur, Kerala, South India. ABSTRACT Plants are valuable source of the therapeutic agents in the armory of modern medicine. The method of drug development from plant sources is based on a sequence of operation leading mainly toward the isolation of pure natural products. An antioxidant is a molecule that inhibits the oxidation of other molecules. Antioxidants have been investigated for the prevention of diseases such as cancer, coronary heart disease and even altitude sickness. The major sources of anti-oxidants are reported to be from the natural source, especially from plant source. Key words: Anti oxidant, Free radical, Medicinal Plants INTRODUCTION pharmacological studies to ascertain their Till date as such no set definition of the term therapeutic properties. (Bakru H.K. 1992). antioxidant exists. Scientists are still striving In this review an attempt has been made o hard to find out the role of particular dietary compile most of the Natural Herbs which supplements in body that have potent health possess ant oxidative property. Following are benefits. Since, different antioxidant the list of such medicinal herbs which are compounds found in diet considerably vary reported to be an antioxidant. -

Biosynthesis of Natural Products
63 2. Biosynthesis of Natural Products - Terpene Biosynthesis 2.1 Introduction Terpenes are a large and varied class of natural products, produced primarily by a wide variety of plants, insects, microoroganisms and animals. They are the major components of resin, and of turpentine produced from resin. The name "terpene" is derived from the word "turpentine". Terpenes are major biosynthetic building blocks within nearly every living creature. Steroids, for example, are derivatives of the triterpene squalene. When terpenes are modified, such as by oxidation or rearrangement of the carbon skeleton, the resulting compounds are generally referred to as terpenoids. Some authors will use the term terpene to include all terpenoids. Terpenoids are also known as Isoprenoids. Terpenes and terpenoids are the primary constituents of the essential oils of many types of plants and flowers. Essential oils are used widely as natural flavor additives for food, as fragrances in perfumery, and in traditional and alternative medicines such as aromatherapy. Synthetic variations and derivatives of natural terpenes and terpenoids also greatly expand the variety of aromas used in perfumery and flavors used in food additives. Recent estimates suggest that over 30'000 different terpenes have been characterized from natural sources. Early on it was recognized that the majority of terpenoid natural products contain a multiple of 5C-atoms. Hemiterpenes consist of a single isoprene unit, whereas the monoterpenes include e.g.: Monoterpenes CH2OH CHO CH2OH OH Myrcens -

DIFFERENCES in Terpenoid Levels Between Plant Species Are Known To
GENETICS OF TEP.PENES I. GENE CONTROL OF MONOTERPENE LEVELS IN PINUS MONTICOLA DOUGL. JAMES W. HANOVER Geneticist, Forestry Sciences Laboratory, lntermountain Forest and Range Experiment Station, U.S. Department of Agriculture, Moscow, Idaho * Receivedi o.v.6 1.INTRODUCTION DIFFERENCESin terpenoid levels between plant species are known to exist but little is known about their genetic bases. The terpenes have been used extensively in biochemical systematic studies in Pinus (Mirov, 1958, 1961; Williams and Bannister, 1962; Forde, 1964), Eucalyptus (Baker and Smith, 1920; Penfold and Morrison, 1927), Cup ressacee (Erdtman, 1958),andmany other plant families and genera (Alston and Turner, 1963).Thesestudies were usually based upon the assumption that variation within a species is relatively small. Although this may be valid in some cases, Bannister et al. (1962)andothers have shown that the level of terpenes can vary with geographic origin within a species. Recent data on tree-to-tree variability in the monoterpenes of Pinus ponderosa Laws. show that such variation can be relatively large (Smith, 1964). Results of similar work in our laboratory with western white pine (Pinus monticola Dougi.) also revealed substantial qualitative and quantitative variation in the cortex monoterpenes between trees. In order to provide a basis for the use of terpenes for comparative biochemical studies, an understanding of both their variability and mode of inheritance is essential. The objective of the present study is to demonstrate the degree to which levels of six monoterpene compounds are gene-controlled in western white pine. Interrelations among the terpenes and between terpenes and growth are also considered, 2.MATERIALS AND METHODS Thematerials for this study are of three types: (i) parents—as clonal lines of grafts, () F1 progeny from crosses between the ortets (parents) represented by the clones, and () S1 progeny of the parent trees. -

Antinociceptive Activity and Redox Profile of the Monoterpenes
2 ISRN Toxicology well as in medicinal plants with a therapeutic property [7– 2.2. Animals. Adult male albino Swiss mice (28–34 g) were 10]. randomly housed in appropriate cages at C with a Recent works have demonstrated that monoterpenes 12/12-h light/dark cycle (light from 06:00 to 18:00),∘ with may present important pharmacological properties includ- free access to food (Purina, Brazil) and tap21 water. ± 2 We used ing antimicrobial [11], antioxidant [3], analgesic [12], and 6–8 animals in each group. Nociceptive tests were carried antitumoral [9] activities, as well as effects on cardiovascular out by the same visual observer and all efforts were made system [13] and central nervous system (CNS) [14]. (+)- to minimize the number of animals used as well as any camphene, p-cymene, and geranyl acetate (Figure 1) are discomfort. Experimental protocols were approved by the monoterpenes present in the essential oils of various plant Animal Care and Use Committee (CEPA/UFS no. 26/09) at species, such as Cypress, Origanum, and Eucalyptus oils [15, the Federal University of Sergipe. 16]. ese substances are present at signi�cant amounts in a wide variety of products derived from natural sources used as food, medicines, or other purposes in different countries. 2.3. Acetic Acid-Induced Writhing. We followed the proce- However, reports with reference to their therapeutic effects dure by Koster et al. [21]. Mice ( , per group) were by studies aiming to establish their individual characteristics, pretreated either by (+)-camphene, p-cymene, or geranyl as described in the present work, are scarce in literature. -

Living Polymerization of Renewable Vinyl Monomers Into Bio-Based Polymers
Polymer Journal (2015) 47, 527–536 & 2015 The Society of Polymer Science, Japan (SPSJ) All rights reserved 0032-3896/15 www.nature.com/pj FOCUS REVIEW Controlled/living polymerization of renewable vinyl monomers into bio-based polymers Kotaro Satoh1,2 In this focused review, I present an overview of our recent research on bio-based polymers produced by the controlled/living polymerization of naturally occurring or derived renewable monomers, such as terpenes, phenylpropanoids and itaconic derivatives. The judicious choice of initiating system, which was borrowed from conventional petrochemical monomers, not only allowed the polymerization to proceed efficiently but also produced well-defined controlled/living polymers from these renewable monomers. We were able to find several controlled/living systems for renewable monomers that resulted in novel bio-based polymers, including a cycloolefin polymer, an AAB alternating copolymer with an end-to-end sequence, a phenolic and high-Tg alternating styrenic copolymer, and an acrylic thermoplastic elastomer. Polymer Journal (2015) 47, 527–536; doi:10.1038/pj.2015.31; published online 13 May 2015 INTRODUCTION aliphatic olefins and styrenes,22,23 whereas the latter applies to most Bio-based polymers are attractive materials from the standpoints of unsaturated compounds bearing C = Cbonds.24–38 Controlled/living being environmentally benign and sustainable. They are usually radical polymerization can precisely control the molecular weights and derived from renewable bio-based feedstocks, such as starches, plant the terminal groups of numerous monomers and has opened a new oils and microbiota, as an alternative to traditional polymers from field of precision polymer synthesis that has been applied to the fossil resources.1 Most of the bio-based polymers produced in the production of a wide variety of functional materials based on 1990s were polyesters prepared via condensation or ring-opening controlled polymer structures. -

Leaf and Flower Volatile Oil Components of Two Thyme Taxa Origanum Onites L
Avrupa Bilim ve Teknoloji Dergisi European Journal of Science and Technology Sayı 17, S. 346-350, Aralık 2019 No. 17, pp. 346-350, December 2019 © Telif hakkı EJOSAT’a aittir Copyright © 2019 EJOSAT Araştırma Makalesi www.ejosat.com ISSN:2148-2683 Research Article Leaf and Flower Volatile Oil Components of Two Thyme Taxa Origanum onites L. and Thymbra spicata var. spicata L. in Turkey Ayşe Gül Sarıkaya1* 1 Bursa Technical University, Faculty of Forestry, Bursa-Turkey (ORCID: 0000-0002-0641-4445) (İlk Geliş Tarihi 10 Eylül 2019 ve Kabul Tarihi 14 Ekim 2019) (DOI: 10.31590/ejosat.618187) ATIF/REFERENCE: Sarıkaya, A. (2019). Leaf and Flower Volatile Oil Components of Two Thyme Taxa Origanum onites L. and Thymbra spicata var. spicata L. in Turkey. Avrupa Bilim ve Teknoloji Dergisi, (17), 346-350. Abstract Medicinal and aromatic plants have a special importance with volatile oil components. Lamiaceae family members are important in pharmacology and perfumery industry because they contain volatile and aromatic oil. Origanum onites L. and Thymbra spicata var. spicata L. are the most widely used and most exported species. The volatile components of the leaves and flowers of Origanum onites L. and Thymbra spicata var. spicata taxa were determined by Headspace Solid Phase Microextraction (HS-SPME) technique combined with gas chromatography/mass spectrometry (GC / MS). 33 different components of Origanum onites were identified and the main components were p-cymene (11.45%), γ-terpinene (11.89%), linalool (14.35%), thymol (20.03%) and carvacrol (26.91%), respectively. For Thymbra spicata var. spicata L., 36 different compounds were identified and the main components were p-cymene (11.72%), γ-terpinene (10.96%), linalool (13.44%), thymol (18.92%) and carvacrol (27.34%), respectively. -

A Genomic Approach to Characterization of the Citrus Terpene Synthase Gene Family
Genetics and Molecular Biology, 30, 3 (suppl), 832-840 (2007) Copyright by the Brazilian Society of Genetics. Printed in Brazil www.sbg.org.br Research Article A genomic approach to characterization of the Citrus terpene synthase gene family Marcelo Carnier Dornelas and Paulo Mazzafera Departamento de Fisiologia Vegetal, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, SP, Brazil. Abstract Terpenes are a very large and structurally diverse group of secondary metabolites which are abundant in many es- sential oils, resins and floral scents. Additionally, some terpenes have roles as phytoalexins in plant-pathogen rela- tionships, allelopathic inhibitors in plant-plant interactions, or as airborne molecules of plant-herbivore multitrophic signaling. Thus the elucidation of the biochemistry and molecular genetics of terpenoid biosynthesis has paramount importance in any crop species. With this aim, we searched the CitEST database for clusters of expressed sequence tags (ESTs) coding for terpene synthases. Herein is a report on the identification and in silico characterization of 49 putative members of the terpene synthase family in diverse Citrus species. The expression patterns and the possible physiological roles of the identified sequences are also discussed. Key words: secondary metabolism, flavor, aroma, plant protection, terpene synthesis. Received: July 21, 2006; Accepted: April 2, 2007. Introduction respectively. Polyterpenes are all terpenes containing more Terpenes are found widely distributed in the plant than eight isoprene units, which include all natural rubbers kingdom, from lichens and algae to higher plants. Although (Goodwin, 1967; Croteau et al., 2000). terpenes cover a wide range of compounds with diverse Elucidation of the biochemistry and molecular genet- structure, the word terpene has been frequently associated ics of terpenoid biosynthesis has made rapid progress in re- with essential oils, which are volatile compounds belong- cent years (Rohdich et al., 2005). -

Salvia Officinalis L. from Italy: a Comparative Chemical And
molecules Article Salvia officinalis L. from Italy: A Comparative Chemical and Biological Study of Its Essential Oil in the Mediterranean Context Rosa Tundis 1,* , Mariarosaria Leporini 1, Marco Bonesi 1, Simone Rovito 2 and Nicodemo G. Passalacqua 2 1 Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, 87036 Rende, CS, Italy; [email protected] (M.L.); [email protected] (M.B.) 2 History Museum of Calabria and Botanic Garden, University of Calabria, 87036 Rende, CS, Italy; [email protected] (S.R.); [email protected] (N.G.P.) * Correspondence: [email protected]; Tel.: +39-0984-493246 Received: 21 November 2020; Accepted: 9 December 2020; Published: 10 December 2020 Abstract: Salvia officinalis L. (sage) is one of the most appreciated plants for its plethora of biologically active compounds. The objective of our research was a comparative study, in the Mediterranean context, of chemical composition, anticholinesterases, and antioxidant properties of essential oils (EOs) from sage collected in three areas (S1–S3) of Southern Italy. EOs were extracted by hydrodistillation and analyzed by gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS). Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory properties were investigated by employing Ellman’s method. Four in vitro assays, namely, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,20-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), ferric-reducing ability power (FRAP), and β-carotene bleaching tests, were used to study the antioxidant effects. Camphor (16.16–18.92%), 1,8-cineole (8.80–9.86%), β-pinene (3.08–9.14%), camphene (6.27–8.08%), and α-thujone (1.17–9.26%) are identified as the most abundant constituents. -

Monoterpenes in the Glandular Trichomes of Tomato Are Synthesized
Monoterpenes in the glandular trichomes of tomato SEE COMMENTARY are synthesized from a neryl diphosphate precursor rather than geranyl diphosphate Anthony L. Schilmillera,1, Ines Schauvinholdb,1, Matthew Larsonc, Richard Xub, Amanda L. Charbonneaua, Adam Schmidtb, Curtis Wilkersona,d, Robert L. Lasta,d, and Eran Picherskyb,2 aDepartments of Biochemistry and Molecular Biology and dPlant Biology, and cBioinformatics Core, Research Technology Support Facility, Michigan State University, East Lansing, MI 48824-1319; and bDepartment of Molecular, Cellular, and Developmental Biology, University of Michigan, Ann Arbor, MI 48109-1048 Communicated by Anthony R. Cashmore, University of Pennsylvania, Philadelphia, PA, April 20, 2009 (received for review December 19, 2008) We identified a cis-prenyltransferase gene, neryl diphosphate (FPP), and diterpene synthases use the C20-diphosphate inter- synthase 1 (NDPS1), that is expressed in cultivated tomato (Sola- mediate E,E,E-geranylgeranyl diphosphate (GGPP) (7). The num lycopersicum) cultivar M82 type VI glandular trichomes and enzymes that synthesize these intermediates are designated as encodes an enzyme that catalyzes the formation of neryl diphos- trans-prenyltransferases, and they consist of a family of struc- phate from isopentenyl diphosphate and dimethylallyl diphos- turally related proteins with representatives found in all branches phate. mRNA for a terpene synthase gene, phellandrene synthase of life (8). 1 (PHS1), was also identified in these glands. It encodes an enzyme In contrast, the isoprene units of some long-chain plant that uses neryl diphosphate to produce -phellandrene as the terpenoids such as rubber and dolichols (the latter group of major product as well as a variety of other monoterpenes. The compounds is present in bacteria, fungi, and animals as well) are profile of monoterpenes produced by PHS1 is identical with linked to each other in the cis (Z) conformation (9, 10). -

Effect of Nanoencapsulation on Volatile Constituents, And
www.nature.com/scientificreports OPEN Efect of nanoencapsulation on volatile constituents, and antioxidant and anticancer activities of Algerian Origanum glandulosum Desf. essential oil Hatem Ali1,2, Abdel Rahman Al-Khalifa1, Abdelhakim Aouf3, Habiba Boukhebti4 & Amr Farouk5* Nanoencapsulation is an attractive novel technique used for incorporating essential oils in food preparations and pharmaceutical formulae. This study investigated the efect of nanoencapsulation on the composition of volatile compounds, as well as the antioxidant and anticancer activities of hydrodistilled (HD) Origanum glandulosum Desf. Oil, which was encapsulated into nanocapsules via High Speed Homogenization (HSH) and into nanoemulsions through High Pressure Homogenization (HPH). Thirty-two volatile components were identifed using Gas Chromatography-Mass Spectrometry analysis (GC-MS) in HD essential oil representing 99.04% of the total oil content. GC-MS analysis showed that the use of HPH to prepare nanoemulsions negatively afected the active compounds present in HD oil, particularly carvacrol and thymol, whereas the use of HSH led to signifcant quantitative diferences in the composition of volatiles between HD oil and nanocapsules but generated the same profle. Consistent with the diferences in total phenolics, total favonoids, and volatiles identifed in HD and nanoparticles, HD essential oil exhibited a higher antioxidant activity (IC50 4.22 mg/ mL) than nanocapsules (IC50 57.51 mg/mL) and nanoemulsion (IC50 78.50 mg/mL), while nanocapsules showed the strongest cytotoxic efect on liver cancer cell line Hep-G2 (54.93 μg/mL) in comparison to HD oil (73.13 μg/mL) and nanoemulsions (131.6 μg/mL). Te Origanum genus (Lamiaceae family) includes approximately 38 species that have been studied extensively for potential importance, and uses in favoring foods and traditional medicine due to their pharmacological charac- teristics1. -
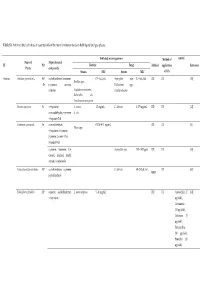
Table S1. Antimicrobial Activities of Essential Oils of the Main Functional Foods in Both Liquid and Gas Phases
Table S1. Antimicrobial activities of essential oils of the main functional foods in both liquid and gas phases Inhibited microorganisms Method of control Name of Major chemical PF PO Bacteria Fungi Method application Reference Plants compounds Strains MIC Strains MIC of EOs Apiaceae Anethum graveolens L. AP, α-phellandrene, limonene, 0.5‒5μL/mL Aspergillus nige, 0.5‒5μL/mL DD DC [58] Bacillus spp., Fr p-cymene, carvone, Trichoderma spp., dillether Staphylococcus aureus, Candida albicans Escherichia coli, Pseudomonas aerogenosa Bunium persicum Fr γ-terpinene, S. aureus, 1.5 mg/mL C. albicans 0.375 mg/mL DD DC [12] cuminaldehyde, p-cymene, E. coli γ-terpinen-7-al Cuminum cyminum L. Se cuminlaldehyde, 0.078–0.31 mg/mL DD DC [8] Vibrio spp. γ-terpinene, o-cymene, β-pinene, 2-caren-10-al, 3-caren-10-al α-pinene, limonene, 1,8- Aspergillus spp. 750‒1000 ppm DD DC [59] cineole, linalool, linalyl acetate, α-terpineole Echinophora Cinerea Boiss AP α-phellandrene, α-pinene, C. albicans 60‒250 μL/mL DC [60] MBD β-phellandrene Echinophora platyloba AP ocimene, α-phellandrene, L. monocytogenes 5‒10 mg/mL DD DC Amoxicillin 25 [61] γ-terpinene μg/disk), Gentamicin (10 μg/disk), Cefexime (5 μg/disk), Tetracycline (30 μg/disk), Penicillin (10 μg/disk) ocimene, 2,3-dimethyl-1,3- 0.63‒0.31 DD DC [62] A. flavus, Penicilium cyclohexadiene, α–pinene, mg./mL γ-dodecalactone expansum, Fusarium graminearum Foeniculum vulgare Mill. Se Shigella dysenteriae 0.125 mg/mL Oc DC [48] trans-anethole, estragole, limonene, fenchone estragole, limonene, 0.16–0.2 DD DC [63] C.