Hedgehog Signaling, a Critical Pathway Governing the Development and Progression of Hepatocellular Carcinoma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Investigator Initiated Study IRB-29839 an Open-Label Pilot Study To
Investigator Initiated Study IRB-29839 An open-label pilot study to evaluate the efficacy and safety of a combination treatment of Sonidegib and BKM120 for the treatment of advanced basal cell carcinomas Version 05 September 2016 NCT02303041 DATE: 12Dec2018 1 Coordinating Center Stanford Cancer Center 875 Blake Wilbur Drive Stanford, CA 94305 And 450 Broadway, MC 5334 Redwood City, CA 94603 Protocol Director and Principal Investigator Anne Lynn S Chang, MD, Director of Dermatological Clinical Trials 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Co-Investigator Anthony Oro, MD PhD 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Biostatistician Shufeng Li, MS 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Study Coordinator Ann Moffat 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] 2 Table of Contents 1 Background ................................................................. 7 1.1 Disease Background ..................................................... 7 1.2 Hedgehog Pathway and mechanism of action ............................... 7 1.3 PI3K Pathway and mechanism of action ................................... 9 1.4 Sonidegib Compound Information ............ Error! Bookmark not defined. 1.4.1 Preclinical Studies for Sonidegib ....................................................................11 1.4.2 Muscular system...............................................................................................13 1.4.3 Skeletal system ................................................................................................13 -

Functional Analysis of the Homeobox Gene Tur-2 During Mouse Embryogenesis
Functional Analysis of The Homeobox Gene Tur-2 During Mouse Embryogenesis Shao Jun Tang A thesis submitted in conformity with the requirements for the Degree of Doctor of Philosophy Graduate Department of Molecular and Medical Genetics University of Toronto March, 1998 Copyright by Shao Jun Tang (1998) National Library Bibriothèque nationale du Canada Acquisitions and Acquisitions et Bibiiographic Services seMces bibliographiques 395 Wellington Street 395, rue Weifington OtbawaON K1AW OttawaON KYAON4 Canada Canada The author has granted a non- L'auteur a accordé une licence non exclusive licence alIowing the exclusive permettant à la National Library of Canada to Bibliothèque nationale du Canada de reproduce, loan, distri%uteor sell reproduire, prêter' distribuer ou copies of this thesis in microform, vendre des copies de cette thèse sous paper or electronic formats. la forme de microfiche/nlm, de reproduction sur papier ou sur format électronique. The author retains ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantial extracts fkom it Ni la thèse ni des extraits substantiels may be printed or otherwise de celle-ci ne doivent être imprimés reproduced without the author's ou autrement reproduits sans son permission. autorisation. Functional Analysis of The Homeobox Gene TLr-2 During Mouse Embryogenesis Doctor of Philosophy (1998) Shao Jun Tang Graduate Department of Moiecular and Medicd Genetics University of Toronto Abstract This thesis describes the clonhg of the TLx-2 homeobox gene, the determination of its developmental expression, the characterization of its fiuiction in mouse mesodem and penpheral nervous system (PNS) developrnent, the regulation of nx-2 expression in the early mouse embryo by BMP signalling, and the modulation of the function of nX-2 protein by the 14-3-3 signalling protein during neural development. -

Role of Sonic Hedgehog Signaling Pathway in Neuroblastoma Development
Review Article Biology and Medicine, 1 (4): Rev2, 2009 eISSN: 09748369, www.biolmedonline.com Role of Sonic hedgehog signaling pathway in neuroblastoma development Mehdi Hayat Shahi1,2,3,§, Subrata Sinha2, *Mohammad Afzal3, *Javier S Castresana1 1Unidad de Biologia de Tumoures Cerebrales, Universidad de Navarra, 31008 Pamplona, Spain. 2Department of Biochemistry, All India Institute of Medical Sciences (AIIMS), New Delhi-110029, India. 3Section of Genetics, Department of Zoology, Aligarh Muslim University, Aligarh-202002, India. §Present address: Department of Genetics and Pathology, Rudbeck Laboratory, University of Uppsala, Uppsala- 75185, Sweden. *Corresponding Authors: Javier S Castresana, [email protected] Mohammad Afzal, [email protected] Abstract Malignant transformation of normal cells is a complex and accumulative process. Understanding this event gives insight into mechanisms of developmental biology and physical interaction of cellular machinery with surrounding ambient factors. However, the trend of embryonic malignancy is not interactive with ambient factors, rather a cause of deregulations of internal developmental process. In this review, we have attempted to explore the possibility of Sonic hedgehog role (Shh) in the development of neuroblastoma tumour. It is the major extra cranial tumour and develops in very early stage of childhood. Sonic hedgehog signaling is very well studied in another major childhood tumour i.e. medulloblastoma that contributes 20-25% of childhood tumours, and one-fourth of medulloblastoma is due to abnormality in the Shh signaling pathway. Therefore, we would consider whether Shh could also contribute to the development of neuroblastoma. Although scientists are coming up with the role of Shh in the neuroblastoma, the Sonic hedgehog signaling is very much one of the promising pathways because of its multi-dimensional role not only in CNS development but also in organogenesis and other major tumour development. -

Sonic Hedgehog Signaling Limits Atopic Dermatitis Via Gli2-Driven Immune Regulation
Sonic Hedgehog signaling limits atopic dermatitis via Gli2-driven immune regulation Eleftheria Papaioannou, … , Ryan F. L. O’Shaughnessy, Tessa Crompton J Clin Invest. 2019. https://doi.org/10.1172/JCI125170. Research Article Immunology Inflammation Hedgehog (Hh) proteins regulate development and tissue homeostasis, but their role in atopic dermatitis (AD) remains unknown. We found that on induction of mouse AD, Sonic Hedgehog (Shh) expression in skin, and Hh pathway action in skin T cells were increased. Shh signaling reduced AD pathology and the levels of Shh expression determined disease severity. Hh-mediated transcription in skin T cells in AD-induced mice increased Treg populations and their suppressive function through increased active transforming growth factor–b (TGF-b) in Tregs signaling to skin T effector populations to reduce disease progression and pathology. RNA sequencing of skin CD4+ T cells from AD-induced mice demonstrated that Hh signaling increased expression of immunoregulatory genes and reduced expression of inflammatory and chemokine genes. Addition of recombinant Shh to cultures of naive human CD4+ T cells in iTreg culture conditions increased FOXP3 expression. Our findings establish an important role for Shh upregulation in preventing AD, by increased Gli-driven Treg cell–mediated immune suppression, paving the way for a potential new therapeutic strategy. Find the latest version: http://jci.me/125170/pdf The Journal of Clinical Investigation RESEARCH ARTICLE Sonic Hedgehog signaling limits atopic dermatitis via Gli2-driven immune regulation Eleftheria Papaioannou,1 Diana C. Yánez,1,2 Susan Ross,1 Ching-In Lau,1 Anisha Solanki,1 Mira Manilal Chawda,1 Alex Virasami,1 Ismael Ranz,3 Masahiro Ono,1,4 Ryan F. -

Products for Morphogen Research
R&D Systems Tools for Cell Biology Research™ Products for Morphogen Research BMP-4 NEURAL PLATE BMP-7 PROSPECTIVE NEURAL CREST NON-NEURAL ECTODERM Noggin Shh Noggin PRESOMITIC MESODERM NOTOCHORD NON-NEURAL ECTODERM FUTURE FLOOR PLATE BMP-4 Shh Noggin DORSAL AORTA ROOF PLATE Products for Morphogen Research for Products Noggin Wnt-1 Wnt-3a Wnt-4 NT-3 Wnt-6 Wnt-7a EARLY SOMITE Myf5 NEURAL TUBE Pax3 Sim1 BMP-4 INTERMEDIATE MESODERM Shh ShhShh NogginNoggin BMP-4 DORSAL AORTA Ihh NOTOCHORD MORPHOGENS Morphogens are molecules that regulate cell fate during development. Formation of morphogen concentration gradients directs the biological responses of surrounding cells. Graded responses occur as a result of morphogens binding to specific cell surface receptors that subsequently activate intracellular signaling pathways and promote or repress gene expression at specific threshold concentrations. Activation or inactivation of these signaling pathways provides positional information that ultimately determines tissue organization and morphology. Research in model organisms has revealed that morphogens are involved in many aspects of development. For example, morphogens are required in Drosophila for patterning of the dorso-ventral and anterior-posterior axes, segment patterning, and positional signaling in the leg and wing imaginal discs. Proteins belonging to the Wingless/Wnt, Notch, Hedgehog, and TGF-b families have been identified as morphogens that direct a number of these processes. Research in higher organisms has demonstrated that homologues of these same signaling molecules regulate vertebrate axis formation, anterior/posterior polarity during limb development, mesoderm patterning, and numerous other processes that establish an organism’s basic body structure. R&D Systems offers a wide selection of proteins, antibodies, and ELISAs for morphogen-related developmental research. -

ABCG1 (ABC8), the Human Homolog of the Drosophila White Gene, Is a Regulator of Macrophage Cholesterol and Phospholipid Transport
ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport Jochen Klucken*, Christa Bu¨ chler*, Evelyn Orso´ *, Wolfgang E. Kaminski*, Mustafa Porsch-Ozcu¨ ¨ ru¨ mez*, Gerhard Liebisch*, Michael Kapinsky*, Wendy Diederich*, Wolfgang Drobnik*, Michael Dean†, Rando Allikmets‡, and Gerd Schmitz*§ *Institute for Clinical Chemistry and Laboratory Medicine, University of Regensburg, 93042 Regensburg, Germany; †National Cancer Institute, Laboratory of Genomic Diversity, Frederick, MD 21702-1201; and ‡Departments of Ophthalmology and Pathology, Columbia University, Eye Research Addition, New York, NY 10032 Edited by Jan L. Breslow, The Rockefeller University, New York, NY, and approved November 3, 1999 (received for review June 14, 1999) Excessive uptake of atherogenic lipoproteins such as modified low- lesterol transport. Although several effector molecules have been density lipoprotein complexes by vascular macrophages leads to proposed to participate in macrophage cholesterol efflux (6, 9), foam cell formation, a critical step in atherogenesis. Cholesterol efflux including endogenous apolipoprotein E (10) and the cholesteryl mediated by high-density lipoproteins (HDL) constitutes a protective ester transfer protein (11), the detailed molecular mechanisms mechanism against macrophage lipid overloading. The molecular underlying cholesterol export in these cells have not yet been mechanisms underlying this reverse cholesterol transport process are characterized. currently not fully understood. To identify effector proteins that are Recently, mutations of the ATP-binding cassette (ABC) trans- involved in macrophage lipid uptake and release, we searched for porter ABCA1 gene have been causatively linked to familial HDL genes that are regulated during lipid influx and efflux in human deficiency and Tangier disease (12–14). -

Ncomms6419.Pdf
ARTICLE Received 6 Jun 2014 | Accepted 29 Sep 2014 | Published 7 Nov 2014 DOI: 10.1038/ncomms6419 OPEN Mechanistic determinants of the directionality and energetics of active export by a heterodimeric ABC transporter Nina Grossmann1,*, Ahmet S. Vakkasoglu2,*, Sabine Hulpke1, Rupert Abele1, Rachelle Gaudet2 & Robert Tampe´1,3 The ATP-binding cassette (ABC) transporter associated with antigen processing (TAP) participates in immune surveillance by moving proteasomal products into the endoplasmic reticulum (ER) lumen for major histocompatibility complex class I loading and cell surface presentation to cytotoxic T cells. Here we delineate the mechanistic basis for antigen translocation. Notably, TAP works as a molecular diode, translocating peptide substrates against the gradient in a strict unidirectional way. We reveal the importance of the D-loop at the dimer interface of the two nucleotide-binding domains (NBDs) in coupling substrate translocation with ATP hydrolysis and defining transport vectoriality. Substitution of the conserved aspartate, which coordinates the ATP-binding site, decreases NBD dimerization affinity and turns the unidirectional primary active pump into a passive bidirectional nucleotide-gated facilitator. Thus, ATP hydrolysis is not required for translocation per se, but is essential for both active and unidirectional transport. Our data provide detailed mechanistic insight into how heterodimeric ABC exporters operate. 1 Institute of Biochemistry, Biocenter, Goethe-University Frankfurt, Max-von-Laue-Street 9, D-60438 Frankfurt/M., Germany. 2 Department of Molecular and Cellular Biology, Harvard University, 52 Oxford Street, Cambridge, Massachusetts 02138, USA. 3 Cluster of Excellence Frankfurt—Macromolecular Complexes, Goethe-University Frankfurt, Max-von-Laue-Street 9, D-60438 Frankfurt/M., Germany. * These authors contributed equally to this work. -

Rapid Changes in Morphogen Concentration Control Self-Organized
RESEARCH COMMUNICATION Rapid changes in morphogen concentration control self-organized patterning in human embryonic stem cells Idse Heemskerk1†, Kari Burt1, Matthew Miller1, Sapna Chhabra2, M Cecilia Guerra1, Lizhong Liu1, Aryeh Warmflash1,3* 1Department of Biosciences, Rice University, Houston, United States; 2Systems, Synthetic and Physical Biology Program, Rice University, Houston, United States; 3Department of Bioengineering, Rice University, Houston, United States Abstract During embryonic development, diffusible signaling molecules called morphogens are thought to determine cell fates in a concentration-dependent way. Yet, in mammalian embryos, concentrations change rapidly compared to the time for making cell fate decisions. Here, we use human embryonic stem cells (hESCs) to address how changing morphogen levels influence differentiation, focusing on how BMP4 and Nodal signaling govern the cell-fate decisions associated with gastrulation. We show that BMP4 response is concentration dependent, but that expression of many Nodal targets depends on rate of concentration change. Moreover, in a self- organized stem cell model for human gastrulation, expression of these genes follows rapid changes in endogenous Nodal signaling. Our study shows a striking contrast between the specific ways ligand dynamics are interpreted by two closely related signaling pathways, highlighting both the *For correspondence: subtlety and importance of morphogen dynamics for understanding mammalian embryogenesis [email protected] and designing optimized protocols for directed stem cell differentiation. Editorial note: This article has been through an editorial process in which the authors decide how † Present address: Department to respond to the issues raised during peer review. The Reviewing Editor’s assessment is that all of Cell and Developmental the issues have been addressed (see decision letter). -
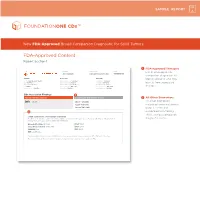
FDA-Approved Content Report Section 1
SAMPLE REPORT New FDA-Approved Broad Companion Diagnostic for Solid Tumors FDA-Approved Content Report Section 1 1 FDA-Approved Therapies PATIENT TUMOR TYPE TRF# List of FDA-approved Jane Sample Lung adenocarcinoma TRFXXXXXX companion diagnostics to PATIENT PHYSICIAN SPECIMEN identify patients who may DISEASE Lung adenocarcinoma ORDERING PHYSICIAN Not Given SPECIMEN SITE Not Given NAME Not Given MEDICAL FACILITY Not Given SPECIMEN ID Not Given benefi t from associated DATE OF BIRTH Not Given ADDITIONAL RECIPIENT Not Given SPECIMEN TYPE Not Given SEX Female MEDICAL FACILITY ID Not Given DATE OF COLLECTION Not Given therapies MEDICAL RECORD # Not Given PATHOLOGIST Not Given SPECIMEN RECEIVED Not Given CDx Associated Findings 1 GENOMIC FINDINGS DETECTED FDA-APPROVED THERAPEUTIC OPTIONS 2 All Other Biomarkers EGFR L858R Gilotrif® (Afatinib) All other biomarkers, Iressa® (Gefitinib) including tumor mutational Tarceva® (Erlotinib) burden (TMB) and 2 microsatellite instability (MSI), without companion OTHER ALTERATIONS & BIOMARKERS IDENTIFIED Results reported in this section are not prescriptive or conclusive for labeled use of any specific therapeutic product. See diagnostic claims professional services section for additional information. Microsatellite Status MS-Stable PTCH1 T416S Tumor Mutation Burden 11 Muts/Mb RBM10 Q494* CDKN2A/B loss TP53 R267P EGFR amplification § Refer to appendix for limitation statements related to detection of any copy number alterations, gene rearrangements, MSI or TMB result in this section. Please refer to appendix -

Genetic Basis of Sjo¨Gren's Syndrome. How Strong Is the Evidence?
Clinical & Developmental Immunology, June–December 2006; 13(2–4): 209–222 Genetic basis of Sjo¨gren’s syndrome. How strong is the evidence? JUAN-MANUEL ANAYA1,2, ANGE´ LICA MARI´A DELGADO-VEGA1,2,& JOHN CASTIBLANCO1 1Cellular Biology and Immunogenetics Unit, Corporacio´n para Investigaciones Biolo´gicas, Medellı´n, Colombia, and 2Universidad del Rosario, Medellı´n, Colombia Abstract Sjo¨gren’s syndrome (SS) is a late-onset chronic autoimmune disease (AID) affecting the exocrine glands, mainly the salivary and lachrymal. Genetic studies on twins with primary SS have not been performed, and only a few case reports describing twins have been published. The prevalence of primary SS in siblings has been estimated to be 0.09% while the reported general prevalence of the disease is approximately 0.1%. The observed aggregation of AIDs in families of patients with primary SS is nevertheless supportive for a genetic component in its etiology. In the absence of chromosomal regions identified by linkage studies, research has focused on candidate gene approaches (by biological plausibility) rather than on positional approaches. Ancestral haplotype 8.1 as well as TNF, IL10 and SSA1 loci have been consistently associated with the disease although they are not specific for SS. In this review, the genetic component of SS is discussed on the basis of three known observations: (a) age at onset and sex-dependent presentation, (b) familial clustering of the disease, and (c) dissection of the genetic component. Since there is no strong evidence for a specific genetic component in SS, a large international and collaborative study would be suitable to assess the genetics of this disorder. -

Annominer Is a New Web-Tool to Integrate Epigenetics, Transcription
www.nature.com/scientificreports OPEN AnnoMiner is a new web‑tool to integrate epigenetics, transcription factor occupancy and transcriptomics data to predict transcriptional regulators Arno Meiler1,3, Fabio Marchiano2,3, Margaux Haering2, Manuela Weitkunat1, Frank Schnorrer1,2 & Bianca H. Habermann1,2* Gene expression regulation requires precise transcriptional programs, led by transcription factors in combination with epigenetic events. Recent advances in epigenomic and transcriptomic techniques provided insight into diferent gene regulation mechanisms. However, to date it remains challenging to understand how combinations of transcription factors together with epigenetic events control cell‑type specifc gene expression. We have developed the AnnoMiner web‑server, an innovative and fexible tool to annotate and integrate epigenetic, and transcription factor occupancy data. First, AnnoMiner annotates user‑provided peaks with gene features. Second, AnnoMiner can integrate genome binding data from two diferent transcriptional regulators together with gene features. Third, AnnoMiner ofers to explore the transcriptional deregulation of genes nearby, or within a specifed genomic region surrounding a user‑provided peak. AnnoMiner’s fourth function performs transcription factor or histone modifcation enrichment analysis for user‑provided gene lists by utilizing hundreds of public, high‑quality datasets from ENCODE for the model organisms human, mouse, Drosophila and C. elegans. Thus, AnnoMiner can predict transcriptional regulators for a studied -

ABCB6 Is a Porphyrin Transporter with a Novel Trafficking Signal That Is Conserved in Other ABC Transporters Yu Fukuda University of Tennessee Health Science Center
University of Tennessee Health Science Center UTHSC Digital Commons Theses and Dissertations (ETD) College of Graduate Health Sciences 12-2008 ABCB6 Is a Porphyrin Transporter with a Novel Trafficking Signal That Is Conserved in Other ABC Transporters Yu Fukuda University of Tennessee Health Science Center Follow this and additional works at: https://dc.uthsc.edu/dissertations Part of the Chemicals and Drugs Commons, and the Medical Sciences Commons Recommended Citation Fukuda, Yu , "ABCB6 Is a Porphyrin Transporter with a Novel Trafficking Signal That Is Conserved in Other ABC Transporters" (2008). Theses and Dissertations (ETD). Paper 345. http://dx.doi.org/10.21007/etd.cghs.2008.0100. This Dissertation is brought to you for free and open access by the College of Graduate Health Sciences at UTHSC Digital Commons. It has been accepted for inclusion in Theses and Dissertations (ETD) by an authorized administrator of UTHSC Digital Commons. For more information, please contact [email protected]. ABCB6 Is a Porphyrin Transporter with a Novel Trafficking Signal That Is Conserved in Other ABC Transporters Document Type Dissertation Degree Name Doctor of Philosophy (PhD) Program Interdisciplinary Program Research Advisor John D. Schuetz, Ph.D. Committee Linda Hendershot, Ph.D. James I. Morgan, Ph.D. Anjaparavanda P. Naren, Ph.D. Jie Zheng, Ph.D. DOI 10.21007/etd.cghs.2008.0100 This dissertation is available at UTHSC Digital Commons: https://dc.uthsc.edu/dissertations/345 ABCB6 IS A PORPHYRIN TRANSPORTER WITH A NOVEL TRAFFICKING SIGNAL THAT