Anemia-Ameliorating Composition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

What Are the Past
Explaining food vocabulary defining relative clauses practice Choose one of the things below and explain what you are thinking of without say any part of its name (so you can’t say “bean” and you can’t say “curd” if you are explaining “bean curd”). Continue explaining until your partner guesses what you are talking about. Useful phrases for defining what something is “It’s an action which…”/ “It’s an action that…” “It’s a place where…”/ “It’s a place which…” “It’s a person/ man/ woman who…”/ “It’s a person/ man/ woman whose…” “It’s a time when…” “It’s a (Chinese/ Japanese) food/ drink/ vegetable/ fruit/ dish/ ingredient/ animal/ tool/ (cooking) implement/ sea creature/ meal which/ that…” “It’s an animal/ sea creature whose…” Vocabulary related to food to define akachochin bar amazake barman bean curd beansprout chawan mushi cha-ya chef chewy rice cake Chinese cabbage/ Chinese lettuce Chinese dumplings Chinese meat bun chuhai cinema (= movie theater) cocktail bar coffee shop conveyor belt sushi bar cream tea crème caramel curry donut daikon dim sum dinner time Easter egg Easter Sunday elevensies English breakfast family restaurant fish eggs G&T girlie bar gobo goya Downloaded from http://tefltastic.wordpress.com green soy beans hanami happoshu happy hour hayashi rice hina matsuri horse hostess bars izakaya Japanese horseradish Japanese-style curry Japanese-style pickles Japanese-style sweets karaoke bar karaoke box kitchen knife Korean barbecue landlady landlord liver lunchtime maid -

(12) Patent Application Publication (10) Pub. No.: US 2014/0050805 A1 Kambayashi Et Al
US 20140050805A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0050805 A1 Kambayashi et al. (43) Pub. Date: Feb. 20, 2014 (54) COMPOSITION, GLUCOSE (30) Foreign Application Priority Data METABOLISM-IMPROVINGAGENT, AND METHOD FOR IMPROVING GLUCOSE Apr. 15, 2011 (JP) ................................. 2011-091461 METABOLISM Publication Classification (75) Inventors: HiroakiO O Kambayashi, Tokyo (JP); (51) A61E36/67Int. Cl. (2006.01) Murakoshi, Tokyo (JP) A613 L/58 (2006.01) A61E36/258 (2006.01) (73) Assignee:-- - - - - Lion Corporation, Tokyo (JP) (52) CPCU.S. Cl................ A61K 36/67 (2013.01); A61K 36/258 (21) Appl. No.: 14/111,576 (2013.01); A61 K3I/51 (2013.01); A61 K3I/58 (2013.01) (22) PCT Filed: Apr. 2, 2012 USPC ........................................... 424/728; 424/734 (57) ABSTRACT (86). PCT No.: PCT/UP2012/058819 A composition, including: (A) Panax notoginseng, and at S371 (c)(1), least one of (B) a Piper longum extract and (C) vitamin B1, a (2), (4) Date: Oct. 14, 2013 salt thereof or a derivative thereof. US 2014/0050805 A1 Feb. 20, 2014 COMPOSITION, GLUCOSE SUMMARY OF INVENTION METABOLISM-IMPROVINGAGENT, AND METHOD FOR IMPROVING GLUCOSE Technical Problem METABOLISM 0010. The present invention aims to solve the above exist ing problems and achieve the following objects. Specifically, TECHNICAL FIELD an object of the present invention is to provide: a composition having excellent glucose metabolism-improving effects, hav 0001. The present invention relates to: a composition suit ing high safety, and capable of being conveniently used, a ably used for improving glucose metabolism; a food, a bev erage, a pharmaceutical drug, a glucose metabolism-improv food, a beverage, a pharmaceutical drug, a glucose metabo ing composition and a glucose metabolism-improving agent, lism-improving composition and a glucose metabolism-im each of which contains the composition; and a method for proving agent, each of which contains this composition; and improving glucose metabolism. -

Izakaya Sushi Noodle Cafe Karaoke Otaku Play Spot Bakery Ramen
japanese sweets 01 天ぷらきよし Tenpura Kiyoshi ★ 01 31Ice cream Baskin Robbins Tenpura restaurant *run by family ice cream 370yen~ 10:30-22:00 Tenpura set menu 850yen~ for lunch fast food ★ izakaya 02/06 不二家 fujiya 12:00-14:30, 17:00-21:00(tue-sun) 01 Freshness Burger Japanese style drinking place. Normally, cake take out 300yen~10:00-21:00 ★ there is a table charge for small dish. 02 /11 松屋 Matsuya Japanese Burger shop, Burgers 350yen~ 03 梅家 Umeya Traditional sweets Gyudon(Beef bowl) many kind dishes Vegie burger,Hot dogs 8:00-23:00 At first, order drink & otsumami from many kind of dishes and enjoy drinking! Many typeJapanese sweets 400yen~ Beef bowl 280yen(M), 24hrs open Season Lunch set 800yen 9:30-20:00 03 Subway Sandwich300yen~ 8:00-21:00 01 ビール工房 Beer Koubou ★ 03 かもん&あっこ kamon&Akko Craft Beer, Food, All 500yen 04 コージーコーナー cozy corner 04/12 モスバーガー Mos Burger cake take out 330yen~ 10:00-22:00 Okonomiyaki/Monjayaki 840yen~ Japaese Burger shop has Rice burgers 18:00-23:00, 15:00-21:00(weekend) 17:00-23:00(mon-sat)16:00-22:00(sun) Mos burger 320yen 9:00-23:00 05 おやき処 れふ亭 Refutei 03 秋吉 Akiyoshi Cheap Yakitori Japanese pancake 120yen~9:00-21:00 04 名代 宇奈とと Unatoto 05/10 McDonalds 24hrs open Negima(Chicken&Green onion) Cheap Unagi(Eel) restaurant ★ 400yen/5sticks 17:00-23:00 07 いさわ Isawa japanese sweets 500yen~ for Unadon 11:00-23:00 06 コロッケ西郷亭 Korokke Saigou Tei Dango110yen take out 11:00-20:30 Croquette 120yen~ Bento boxes 04 馬肉食堂さくら Horsemeat Sakura 05 かつや Katsuya 10:00-21:00,-20:00(sat,sun) Raw horse450yen Grilled horse150yen S ★ Beer 400yen 18:00-23:00 closed sun supermarket Cheap pork cutlet restaurant 07 健康食卓 わしや Washiya Enjoy Japanese supermarket! They have Katsudon 490yen~ 7:00-25:30 Try many Japanese dishes selling by 05 加賀屋 Kagaya old-fashioned Place discount daily food before their closing. -
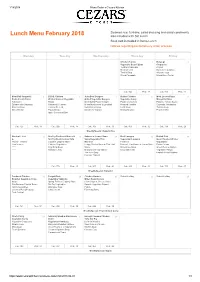
Lunch Menu February 2018 Also Included W Ith Set Lunch Soup Not Included in Bento Lunch Notices Regarding Bento/Delivery Order Process
1/18/2018 Menu Order « Cezars Kitchen Steamed rice, furikake, salad dressing and salad condiments Lunch Menu February 2018 also included w ith Set Lunch Soup not included in Bento Lunch Notices regarding bento/delivery order process Monday Tuesday Wednesday Thursday Friday Chicken Fajitas 1 Bulgogi 2 Vegetable Bean Fajitas Chapuche Tortilla & Salsa Bar Chijimi Mexican Corn Namuru Vegetables Tortilla Soup Wakame soup Sliced Pineapple Mixed Berry Parfait Cal.: 744 Prot.: 27 Cal.: 935 Prot.: 29 Meat Ball Spaghetti 5 B.B.Q. Chicken 6 Juicy Beef Burgers 7 Butter Chicken 8 Meat Lovers Pizza 9 Pesto Cream Pasta W inter Harvest Vegetable Grilled Vegetable Burgers Vegetable Curry Margarita Pizza Ratatouille Roast Oven Baked Potato Wedges Potato Croquettes Pasta w. Tomato Sauce Spinach with Garbanzo Macaroni & Cheese Mixed Mushrooms & Eggplant Roasted Pumpkin Caponata Vegetables Broccoli Soup Corn on the Cob Swiss Onion Soup Lentil Soup Tuscan Soup Sliced Melon Potato Bisque Strawberry Yoghurt Mango Mousse Peach Melba Apple Cinnamon Bake Cal.: 725 Prot.: 38 Cal.: 926 Prot.: 38 Cal.: 706 Prot.: 39 Cal.: 809 Prot.: 32 Cal.: 774 Prot.: 26 W eekly Special: Hayashi Rice Student - Led 12 Stir Fry Chicken & Broccoli 13 Salmon w. Lemon Sauce 14 Beef Lasagna 15 British Fish 16 & Stir Fry Mushroom & Tofu Spicy Vegetable Rice Vegetarian Lasagna Oven Roasted W inter Parent - Teacher Gyoza w. Dipping Sauce Noodles Focaccia Vegetables Conferences Chinese Vegetables Crispy Chicken Bites w. Thai Chili Broccoli, Cauliflower & Carrot Sauté Potato Crisps ***** Egg Drop Soup Sauce Minestrone Soup Green Peas w. Onions ***** Mandarin Jelly Mixed Green Vegetables Chocolate Cake Vegetable Potage Tom Yum Soup English Bread Pudding Coconut Tapioca Cal.: 778 Prot.: 23 Cal.: 807 Prot.: 42 Cal.: 853 Prot.: 32 Cal.: 733 Prot.: 27 W eekly Special: Butadon Tandoori Chicken 19 Teriyaki Pork 20 Chicken Nachos 21 School Holiday 22 School Holiday 23 Roasted Vegetable Cous Vegetable Yakisoba Mixed Bean Nachos Cous Spring Rolls w. -

PDF/PPT You Submitted Via Owlspace Assignments
2014 NanoJapan: International Research Experience for Undergraduates (NSF-PIRE) 4 Program Overview 5 Program Administrators 6 NanoJapan Students 8 Piccell Phone Information 10 Dialing Instructions & ER Phone Numbers 12 Arrival in Houston 13 Rice Campus Map 14 Pre-Departure Orientation 15 International Flight Itinerary 16 Travel to Japan 17 Arrival in Tokyo and Sanuki Club Map 20 Pre-Paid Subway Cards 21 Orientation Schedule in Tokyo 30 Kyushu Trip 33 Orientation Schedule in Tokyo 36 Travel to Research Host Labs 37 Research Internships Part I 38 Mid-Program Meeting in Okinawa with Flight Itineraries 43 Mid-Program Meeting in Okinawa 44 Research Internships Part II 45 Research Symposium in Tokyo & Return to U.S. 46 Re-Entry Program & RQI Symposium 49 Travel Resources and Guides 50 Sanuki Club Rules 51 Money in Japan 52 Food in Japan 57 Transportation in Japan 63 Accommodation & Sight-seeing in Japan 65 Helpful Tokyo Subway Directions 66 Tokyo JR Lines and Tokyo Subway Map 68 Directions to Elionix 72 Emergency and Medical Resources 73 International SOS Japan Country Report 88 Medical Care in Japan & CISI Insurance 93 Disaster Preparedness Information 101 Safety Tips Abroad 102 U.S. Dept. of State Students Abroad - Alcohol Abroad 103 U.S. Dept. of State Students Abroad - Victim of a Crime 104 U.S. Dept. of State Students Abroad - Women Travelers 105 Japanese Language Resources 4 / Program Overview This National Science Foundation Partnerships in International Research and Education (NSF-PIRE) grant supports the expansion of a unique interdisciplinary U.S. - Japan research and educational partnership focused on terahertz (THz) dynamics in nanostructures (OISE #0968405). -

Delivery Item List
Meet, Fish / 肉、魚類 (April 13th - April 20th) Sale Sale Brand Descriotion Note QNTY Brand Descriotion Price Note QNTY Price Price Ground Pork / 豚ひき肉 1LB / 454g Frozen GroundChicken / 鶏ひき肉 1LB / 454g Frozen Grorund Beef / 牛ひき肉 1LB / 454g Frozen Sliced Pork / 豚肉薄切り 1LB / 454g Frozen Shabushabu Pork / しゃぶしゃぶ豚肉 1LB / 454g Frozen Sukiyaki Beef / すき焼き用牛肉 1LB / 454g Frozen Chicken Thigh / 鶏モモ肉 2LB / 907g Frozen Salted Salmon Fillet / 塩鮭切り身 3pcs Frozen Horse Mackel / あじ 開き 2pcs Frozen Mackerel Fillet / さば 切り身 2pcs Frozen Atka Mackerel / ほっけ開き Eel Kabayaki / うなぎ蒲焼き 255g Frozen Seasoned Squid / 味付けイカ 2pcs Frozen Immitation Crab Meat / かにかま 8pcs Frozen Spicy Cod Roe Tube / 明太子チューブ 200g Frozen Shishamo / 子持ちししゃも 300g Frozen Breaded Shrimp / エビフライ 6pcs Frozen 1 Frozen Food / 冷凍食品 (April 13th - April 20th) Brand Descriotion Note QNTY Brand Descriotion Note QNTY Shirakiku Frozen Udon / 冷凍うどん 5p OK Sushi Fried Soy Bean Curd Puff / すしあげ 10pcs Kotsubu Natto / 小粒納豆 3p Okame Natto Extra Small / おかめ納豆極小粒 3p Maruki Fried Soy Bean Curd / あぶらあげ 5pcs Gyoza Skins/ 餃子の皮 47 Sheets Wonton Skins / ワンタンの皮 44 Sheets Wel-Pac Premium Edamame Sai / 枝豆 彩 300g Spring Roll Skins / 春巻きの皮 26 Sheets Chamame / 茶豆 400g Hikiwari Natto / ひきわり納豆 3p Lotus Root / れんこんスライス 1LB / 454g Pumpkin / かぼちゃ 1LB / 454g J-Basket Frozen Sanuki Udon / 冷凍讃岐うどん 5p JFC Daifuku Mochi Mame / 豆大福 110g Takoyaki / たこ焼き 18pcs Daifuku Mochi White / 白大福 110g Daifuku Mochi Grass / 草大福 110g Mizkan Less Smell Natto / におわなっとう 3p Daifuku Mochi Red / 赤大福 110g Toromame Natto/ とろっ豆 3p Daifuku Mochi White Sesame / 白胡麻大福 -

International Research Experience for Undergraduates (NSF-PIRE)
2012 NanoJapan: International Research Experience for Undergraduates (NSF-PIRE) 4 Program Overview 5 Program Administrators 6 NanoJapan Students 8 Piccell Phone Information 10 Calling Instructions & ER Phone Numbers 11 2012 Program Schedule 12 Pre-Departure Orientation 16 Travel to Japan 18 Arrival in Tokyo and Sanuki Club Map 20 Orientation Schedule 32 Tohoku Trip to Minami-Sanriku 33 Travel to Research Host Labs 35 Mid-Program Meeting 41 Return to Tokyo & U.S. 43 Re-Entry Program & RQI Symposium 45 Travel Resources and Guides 46 Sanuki Club Rules 47 Money in Japan 48 Budget Travel in Japan 52 Train Etiquette in Japan 53 Helpful Tokyo Subway Directions 54 Tokyo JR & Subway Maps 56 Directions to Elionix 60 Kyoto Walking Tours 66 Emergency & Medical Resources 67 U.S. Dept. of State Japan Country Information Sheet 81 Illness or Accident Abroad 82 Medical Care in Japan & CISI Insurance 84 Int’l SOS & U.S. Dept. of State STEP Registration 85 Disaster Preparedness for Americans in Japan 92 Calling for Help in Japan 90 Safety Post-Fukushima 96 U.S. Dept. of State Students Abroad - Safety Tips 97 U.S. Dept. of State Students Abroad - Alcohol Abroad 98 U.S. Dept. of State Students Abroad - Victim of a Crime 99 U.S. Dept. of State Students Abroad - Women Travelers 100 Japanese Language Resources 4 / Program Overview This National Science Foundation Partnerships in International Research and Education (NSF-PIRE) grant supports the expansion of a unique interdisciplinary U.S. - Japan research and educational partnership focused on terahertz (THz) dynamics in nanostructures (OISE #0968405). As the fields of science and engineering become increasing international there is a pressing need for the development of research and education programs to produce globally aware scientists and engineers. -

Hayashi Rice ハヤシライス
た つく しゅん あじ Let’s Cook and Eat the Tastes of the Season! 食べよう! 作ろう! 旬の味 はやしらいす Hayashi Rice ハヤシライス ------------------------------------------------------------------- ------------------------------------------------------------------------- ざいりょう にんぶん [Ingredients (four servings)] [材料(4人分)] 200 g thinly sliced beef ぎゅううす ぎ にく たま こ はくはん はいぶん 1 onion 牛 薄切り肉…200g 玉ねぎ…1個 白飯…4杯分 ばたー こむぎこ おお 4 bowls white rice バター…40g 小麦粉…大さじ2 40 g butter とまとけちゃっぷ みず 2 Tbsp flour トマトケチャップ…200ml 水…400ml しょうゆ おお しお こしょう しょうしょう 200 ml ketchup 醤油…大さじ2 塩・コショウ…少 々 400 ml water つく かた 2 Tbsp soy sauce [作り方] Salt & pepper ぎゅうにく ひろ しおこしょう こむぎこ 1.牛肉は広げて塩コショウし、小麦粉をまぶしておく。 [Preparation] たま くし ぎ 1.Lightly salt and pepper the slices of beef and dust 2.玉ねぎは櫛切りにしておく。 them with flour. ふらいぱん ぶんりょう はんぶんほど ばたー い や 2. 3.フライパンに分量の半分程のバターをひき、1を入れる。焼 Cut onion into wedges. め つ と だ 3.Add 20 g of butter to a heated frying pan, then place き目が付いたら取り出す。 the slices of beef in the pan. Take the beef out when it つか ふらいぱん のこ ばたー い 2 いた 4.3で使ったフライパンに残りのバターを入れ、2を炒める。 is cooked. ひ とお ふらいぱん もど ま いた 4.Add the remaining butter to the frying pan and 5.4に火が通ったら3をフライパンに戻し、よく混ざるように炒 stir-fry the onion wedges. 5.When the onion wedges are cooked, return the beef める。 けちゃっぷ みず しょうゆ い ま ふた よわび slices to the frying pan and stir-fry them together. 6.5にケチャップ、水、醤油を入れよくかき混ぜ、蓋をして弱火 6.Add ketchup, water, and soy sauce to the frying pan ふんほど に and mix well. Cover the pan with a lid and simmer on で15分程煮る。 さら はくはん うえ か で き あ きざ low heat for about 15 minutes. -

DAILY LUNCH SOBA SET MENU Sea Urchin Soba
DAILY LUNCH SOBA SET MENU * Only 30 Servings per Day 酒蔵ランチ日替わり蕎麦セット ] 12.50 All Daily Specials include Housemade Buckwheat Soba Noodles (Cold or Hot) 8/6/2018 - 8/31/2018 Monday GYU MISOYAKI DON Grilled Beef Marinated in Miso Over Rice Tuesday NIKOMI HAMBURG STEAK 煮込みハンバーグ Stewed Hamburg Steak with Demi-Glace Sauce Served with a Bowl of Rice Wednesday Thursday - Bara Chirashi Don SAKE NO SHIOYAKI 鮭の塩焼き Fatty Salmon Grilled with Salt Served with a Bowl of Rice Thursday BARA CHIRASHI DON 酒蔵名物 バラチラシ丼 Assortment of Raw Fish and Dried Squash Over Rice (*) Friday HAYASHI RICE 酒蔵イチオシ ハヤシライス Friday - Hayashi Rice Hashed Beef in Thick Demi-Glace Sauce Over Rice Sea Urchin Soba (Served Cold) 酒蔵特製 冷製雲丹蕎麦 Housemade Buckwheat Noodles topped with Fresh Sea Urchin Sashimi served with Sea Urchin Soup ( * ) (Only 7 Servings per Day) 23.00 Soba noodles come with bonito-based broth. Please inform your server of any allergies. Consuming raw or undercooked food (*) such as meats, poultry, seafood, shellfish or eggs may increase your risk of foodborne illness. We may add an 18% gratuity to parties of 6 or grater / Maximum four credit cards per table please. Menu subject to change depending on availability. Lunch V ...Vegetarian Items APPETIZERS [前菜] Assorted Sashimi ( * ) Three kinds ...18.00 Five kinds ...28.00 Tempura Assortment Shrimp Only ...13.00 Shrimp & Vegetables ...13.00 Vegetables Only V ...10.00 Kakiage Tempura Chopped Shrimp and Vegetable Tempura 12.00 Gindara Yuan-yaki Grilled Fillet of Black Cod Steeped in Sweet Soy Sauce 16.00 Gomaae V Lightly -
Consulate General of Japan in Miami Recipes Activities Explore Education
Subscribe to our email list Consulate General of Japan in Miami 在 マ イ ア ミ 日 本 国 総 領 事 館 We at the Consulate General of Japan in Miami hope that everyone is staying safe and healthy. Please enjoy our adapted monthly newsletter, featuring both in-person events under strict COVID-19 guidelines, and activities and resources that you can access right from home! Recipes Just One Cookbook - Japanese Souffle Pancakes These fluffy pancakes are extremely popular in Japanese cafes, and can be easily prepared at home! Simply follow along with Chef Nami, a Japanese home cook from California, as she demonstrates the steps to getting a batter with the perfect airy consistency. Looking to add even more Japanese flair to your breakfast? Check out these matcha souffle pancakes too! Cooking With Dog - Hayashi Rice Though considered a "Western-style" meal, this dish is unique to Japan! Join Francis the toy poodle and Chef as they teach you the steps to making this rich, hearty recipe with tender beef, vegetables, and a demi-glace sauce. The stew pairs well with steamed rice and is a comfort food for people of all ages. Activities Children of the Sea June 13 & 15, 2021 When Ruka was younger, she saw a ghost in the water at the aquarium where her dad works. Now she feels drawn toward the aquarium and the two mysterious boys she meets there, Umi and Sora. The latest feature film from Japan’s STUDIO4°C returns to select theaters across the state of Florida for two days only - purchase tickets in advance for these special screenings here! WasabiCon Geek Marketplace June 26, 2021 | 11 am - 7 pm St. -

2.28.2020 Daily Lunch Special Higawari for Menu
Sakagura Lunch Signature Dish Vegetable Soba Gozen [ 野菜蕎麦御膳] 21.00 Avocado sashimi, assorted appetizers, housemade soba noodles HOT or COLD (cold served with sesame sauce) Sashimi Soba Gozen [ 刺身蕎麦御膳] 25.00 3 kinds of sashimi, assorted appetizers, housemade soba noodles HOT or COLD ( * ) “Jewel” Oke Bento [ 旬菜・桶弁当] 25.00 Assorted appetizers, kakiage tempura, 5 kinds of sashimi, grilled tidbits and mini rice balls ( * ) Only 10 servings per day SAKAGURA Lunch Tasting Course [ 前菜] 39.00 Appetizers: 5 kinds of sashimi and appetizer assortment ( * ) with sake pairing Mains: Assorted tempura (shrimp, white fish and vegetables), an additional $22 grilled fish, beef fillet steak, and COLD housemade soba (small) ( * ) Dessert: Choose one scoop of ice cream Consuming raw or undercooked food ( * ) such as meats, poultry, seafood, shellfish or eggs may increase your risk of foodborne illness. We may add 20% gratuity to parties of 5 or greater / Maximum four credit cards per table please. DAILY LUNCH SOBA SET MENU (Only 30 servings per day) [ 酒蔵ランチ日替わり蕎麦セット ] 13.50 Daily Specials include housemade buckwheat soba noodles (Cold or Hot) 2/3/2020 - 2/28/2020 Monday BUTA DON 豚丼 Thinly sliced pork and onions simmered in sake, mirin, and soy sauce and topped with red pickled ginger. Served over rice. Tuesday SABA MISO NI 鯖の味噌煮 Fresh mackerel simmered in a mixed red and white miso base containing ginger. Served with a bowl of rice. Wednesday Thursday - Bara Chirashi Don TORI SOBORO DON 鶏そぼろ丼 Minced seasoned chicken and Japanese-style scrambled eggs. Served over rice. Thursday BARA CHIRASHI DON 酒蔵名物 バラチラシ丼 Assortment of raw fish and dried squash. -

Mixed Reviews for Evacuation Drill
Volume 12 Issue 19 News Desk - Tel: 076-236555 May 7 - 13, 2005 Daily news at www.phuketgazette.net 20 Baht The Gazette is published in association with High anxiety Mixed reviews for as Dulwich talks come IN THIS ISSUE evacuation drill to a close NEWS: Phuket City mayor ducks dismissal debate; La- By Gazette Staff bor Office aims for faster By Anongnat Sartpisut work permits. Pages 2 & 3 & Stephen Fein PHUKET: Teachers and parents of students at Dulwich Interna- INSIDE STORY: Continuing the PATONG: The evacuation drill tional College (DIC) were wait- account of Patong’s first of the southern half of Patong ing anxiously as the Gazette went evacuation drill. Pages 4 & 5 Beach on Friday, April 29 re- to press to learn the outcome of AROUND THE ISLAND: Love on ceived mixed reviews, from both a May 3 meeting of the board of the lagoon, not the rocks, at tourists and the many govern- Dulwich College in London Laguna. Page 6 ment officials who attended. (DCL) which could decide, af- Arriving by helicopter ter weeks of intense negotiations, AROUND THE REGION: Getting shortly after 10 am, Prime Min- whether DIC is allowed to keep the picture in Khao Lak. ister Thaksin Shinawatra met a the “Dulwich” in its name. Page 8 crowd of some 2,000 volunteer Negotiating on behalf of PHUKET PEOPLE: Lisa Loud: evacuees, many of them chil- DCL in talks in Phuket and superstar DJ. Here we go! dren, wearing T-shirts made spe- Bangkok was Ralph Mainard, Pages 10 & 11 cially for the event.