IPRAS Journal Issue 7 CONTENTS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Forest, Resources and People in Bulungan Elements for a History of Settlement, Trade, and Social Dynamics in Borneo, 1880-2000
CIFOR Forest, Resources and People in Bulungan Elements for a History of Settlement, Trade, and Social Dynamics in Borneo, 1880-2000 Bernard Sellato Forest, Resources and People in Bulungan Elements for a History of Settlement, Trade and Social Dynamics in Borneo, 1880-2000 Bernard Sellato Cover Photo: Hornbill carving in gate to Kenyah village, East Kalimantan by Christophe Kuhn © 2001 by Center for International Forestry Research All rights reserved. Published in 2001 Printed by SMK Grafika Desa Putera, Indonesia ISBN 979-8764-76-5 Published by Center for International Forestry Research Mailing address: P.O. Box 6596 JKPWB, Jakarta 10065, Indonesia Office address: Jl. CIFOR, Situ Gede, Sindang Barang, Bogor Barat 16680, Indonesia Tel.: +62 (251) 622622; Fax: +62 (251) 622100 E-mail: [email protected] Web site: http://www.cifor.cgiar.org Contents Acknowledgements vi Foreword vii 1. Introduction 1 2. Environment and Population 5 2.1 One Forested Domain 5 2.2 Two River Basins 7 2.3 Population 9 Long Pujungan District 9 Malinau District 12 Comments 13 3. Tribes and States in Northern East Borneo 15 3.1 The Coastal Polities 16 Bulungan 17 Tidung Sesayap 19 Sembawang24 3.2 The Stratified Groups 27 The Merap 28 The Kenyah 30 3.3 The Punan Groups 32 Minor Punan Groups 32 The Punan of the Tubu and Malinau 33 3.4 One Regional History 37 CONTENTS 4. Territory, Resources and Land Use43 4.1 Forest and Resources 44 Among Coastal Polities 44 Among Stratified Tribal Groups 46 Among Non-Stratified Tribal Groups 49 Among Punan Groups 50 4.2 Agricultural Patterns 52 Rice Agriculture 53 Cash Crops 59 Recent Trends 62 5. -

Head of Regional Investment and Permittance Board of East Kalimantan) Coal Mining Potencies in East Kalimantan Brief Profile of East Kalimantan
PRESENTED BY DIDDY RUSDIANSYAH A.D (HEAD OF REGIONAL INVESTMENT AND PERMITTANCE BOARD OF EAST KALIMANTAN) COAL MINING POTENCIES IN EAST KALIMANTAN BRIEF PROFILE OF EAST KALIMANTAN Total area of Kalimantan Timur is 125.336,81 km square (or 12,726,752 hectares), consists of : - 3 (three) Cities : 1. Samarinda 2. Balikpapan 3. Bontang - 7 (seven) Regencies : 4. Kutai Kartanegara 5. Kutai Timur 6. Kutai Barat 7. Berau 8. Penajam Paser Utara 9. Paser 10. Mahakam Hullu Its population up to 2014 is 3,508 million inhabitants, with the result that the average population density is 26,14 inhabitants/km square REGIONAL GEOLOGY ....... From geological point of view, East Kalimantan is located in three major tertiary sedimentary basins which have major impact on the process of mineral resources formation in the region. The three basins are : Kutai Basin which covers the area of Mahakam Hilir and Mahakam Hulu. Pasir Basin which covers the area of Paser. Tarakan Basin which covers the area of Tarakan, Berau, and Bulungan. COAL BEARING FORMATION Coal Bearing Formations in Kalimantan Timur are : Balikpapan Formation Pulaubalang Formation Pamaluan Formation Kuaro Formation Wahau Formation Batuayau Formation Tanjung Formation Warukin Formation Telakai Formation Birang Formation Latih Formation COAL RESOURCES AND RESERVES IN EAST KALIMANTAN IN 2012 – 2014 Coal Calorie 5000 up to 7000 Ccl and Sulphur 0,8 up to 1,5 Description 2012 2013 2014 Resources 31.817.269.817 32.258.774.367 30.651.444.628 (MT) Reserves 9.244.407.452 9.525.868.005 8.826.730.632 -

Analisis Kesenjangan Pendapatan Kabupaten/Kota Di Wilayah Kalimantan Utara
PENELITIAN DASAR Laporan Hasil Penelitian ANALISIS KESENJANGAN PENDAPATAN KABUPATEN/KOTA DI WILAYAH KALIMANTAN UTARA Peneliti: NURUS SOIMAH, M.Ec.Dev. (Dosen Tetap Fakultas Ekonomi Universitas Kaltara) LEMBAGA PENELITIAN DAN PENGABDIAN MASYARAKAT UNIVERSITAS KALTARA ABSTRAK Tujuan penelitian ini adalah untuk mengetahui tingkat kesenjangan pendapatan antar kabupten/ kota di Wilayah Kalimantan Utara. Data yang digunakan dalam penelitian ini adalah data sekunder yang diterbitkan oleh pemerintah kabupaten/kota di Kalimantan Utara tahun 2013-2019. Penelitian ini dilakukan di 4 kabupaten dan 1 kota di Kalimantan Utara. Alat analisis yang digunakan dalam penelitian ini adalah analisis deskriptif dengan menggunakan peralatan analisis Ekonomi Regional. Analisis data yang digunakan sesuai dengan tujuan dari penelitian ini adalah Analisis Tingkat Ketimpangan Antar Daerah, untuk menghitung tingkat ketimpangan/disparitas pendapatan perkapita antar kabupaten/kota di Provinsi Kalimantan Utara dengan menggunakan alat analisis Indeks Williamson. Hasil analisis dapat disimpulkan adanya ketimpangan pendapatan yang terjadi di kabupaten/kota di Provinsi Kalimantan Utara meskipun tergolong dalam ketimpangan rendah, namun hal ini perlu terus di kontrol mengingat Kota Tarakan memiliki kecenderungan ketimpangan pendapatan yang semakin tinggi. Ketimpangan terendah terjadi di Kabupaten Tana Tidung dan paling tinggi di Kota Tarakan. Saran yang dapat diberikan dari hasil penelitian ini bagi pemerintah daerah Kabupaten/Kota di Kalimantan Utara adalah agar terus mampu membuat kebijakan yang tepat sehingga mampu mempertahankan kesenjangan yang cukup rendah tersebut. Kata Kunci : Kesenjangan Pendapatan, Indeks Williamson ABSTRACT The purpose of this study is to determine the level of income disparity between districts / city in the North Kalimantan. The type of data in this research is secondary data obtained from the published author of districts / cities in north Kalimantan in year 2013-2019. -

East Kalimantan
PROVINCE INFOGRAPHIC EAST KALIMANTAN Nunukan NUNUKAN Tideng Pale Malinau TANA The boundaries and names shown and the TID UNG designations used on this map do not imply KOTA TARAKAN official endorsement or acceptance by the Tarakan United Nations. MA LINAU BULUNGAN Tanjungselor MOST DENSE LEAST DENSE Tanjung Selor Kota Balikpapan Malinau Tanjungredep MOST POPULATED LEAST POPULATED BERA U Kota Samarinda Tana Tidung 14 1,435 KUTAI DISTRICTS VILLAGES TIMUR Putussibau Sangatta 136 KAPU AS Ujoh Bilang HULU SUB-DISTRICTS Bontang SINTANG KOTA MU RUNG KUTAI BONTANG RAYA KARTANEGARA Legend: Sendawar KOTA SAMARIND A Administrative Boundary Tenggarong Samarinda Samarinda Province Province Capital Purukcahu District District Capital BARITO KUTAI GUNUN G UTARA BARAT MA S Population Transportation Muara Teweh PEN AJAM Population counts at 1km resolution Toll road PA SER Kuala Kurun UTARA KOTA Pasangkayu Primary road 0 BALIKPAPAN Secondary road 1 - 5 Balikpapan Port 6 - 25 Penajam BARITO KATINGAN Airport 26 - 50 SELATAN 51 - 100 Buntok KOTA Other KAPU AS TABALONG PASER 101 - 500 PALANGKA Kasongan Volcano 501 - 2,500 RAYA Tanah Grogot Tamiang Water/Lake 2,501 - 5,000 KOTAWARINGIN Layang Tobadak Tanjung 5,000 - 130,000 TIMUR Palangka Raya BARITO Coastline/River TIMUR Palangkaraya Paringin MA MUJU HULU BALANGAN SUNGAI Amuntai TAPIN UTARA Barabai HULU Sampit SUNGAI KOTA PULANG BARITO HULU SUNGAI Mamuju MA MASA SELATAN TEN GAH BARU GEOGRAPHY PISAU KUALA Mamuju TORA JA East Kalimantan is located at 4°24'N - 2°25'S and 113°44' - 119°00'E. The province borders with Malaysia, specifically Sabah and Sarawak (North), the Sulawesi Ocean and Makasar Straits (East), South Kalimantan (South) and West Kalimantan, Central Kalimantan and Malaysia (West). -

The Relationship of Marine Tourism, Fishing Activities, and Conservation Efforts on Derawan Island, Indonesia
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 2018 The Relationship of Marine Tourism, Fishing Activities, and Conservation Efforts on Derawan Island, Indonesia Heva Hayuqo Yumi University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Yumi, Heva Hayuqo, "The Relationship of Marine Tourism, Fishing Activities, and Conservation Efforts on Derawan Island, Indonesia" (2018). Open Access Master's Theses. Paper 1241. https://digitalcommons.uri.edu/theses/1241 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. THE RELATIONSHIP OF MARINE TOURISM, FISHING ACTIVITIES, AND CONSERVATION EFFORTS ON DERAWAN ISLAND, INDONESIA BY HEVA HAYUQO YUMI A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS IN MARINE AFFAIRS UNIVERSITY OF RHODE ISLAND 2018 MASTER OF ARTS IN MARINE AFFAIRS OF HEVA HAYUQO YUMI APPROVED: Thesis Committee: Major Professor Amelia Moore Robert Thompson Austin Humphries Nasser H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2018 ABSTRACT Derawan Island in eastern Indonesia exemplifies how the designation of a new development category called a “Tourism Village” might not be optimal for a small island because of some issues which may be correctable. Derawan was historically a fishing village. Located in the Coral Triangle, the island is known for its unique biodiversity and world-class diving, and today the island relies on marine tourism as its primary livelihood. -

North Kalimantan Province Has Five Districts and One • Malinau : 226.322 Inhabitants City
PROVINCE OVERVIEW INDONESIA INDUSTRIAL ESTATES DIRECTORY 2018-2019 North Kalimantan Province Beautiful beach of Derawan orth Kalimantan is located in the northern part of Kalimantan Island. The capital city is Tanjung Selor. Basic Data North Kalimantan borders the Malaysian states of NSabah to the north and Sarawak to the west, and the Capital: Tanjung Selor Indonesian province of East Kalimantan to the south. North Kalimantan is the newest province of Indonesia, Major Cities: created on the 25th of October 2012. Administratively, • Tarakan : 239.973 inhabitants North Kalimantan province has five districts and one • Malinau : 226.322 inhabitants city. Its population of 738.163 is spread over an area of • Bulongan : 140.567 inhabitants 75.467,70 km2. • Nunukan : 62.460 inhabitants In developing the province, the government has • Tana Tidung : 22.841 inhabitants set the vision to ”harmonize in Pluralism to achieve an 2 independent, safe, peaceful, clean and proud North Size of Province: 72.567.49 km Kalimantan by 2020“. This vision is to be achieved by reducing poverty and unemployment, increasing economic Population: competitiveness of the agroindustry, tourism, and (1) Province : 738.163 inhabitants sustainable mining and by enhancing North Kalimantan’s (2015) human resources quality to become smarter, nobler, more (2) Province Capital : 42.231 (2012) skillful, and highly competitive. Moreover, the government Salary (2018): wants to develop the province’s infrastructure to enhance The provincial monthly minimum wage : interregional connectivity within Indonesia and with USD 189,62. neighboring countries. The dominant economic sectors of North Kalimantan are mining, agriculture, construction, and the processing industry. In mining, North Kalimantan has many products Educational Attainment such as, crude oil, natural gas, coal, and gold, while for Never attending agriculture, the products produced in North Kalimantan DIPLOMA school % are rice, corn, soy, and livestock. -

Peraturan Daerah Kota Tarakan Nomor 3 Tahun 2019 Tentang Hari Jadi Kota Tarakan.Pdf
[.._ __ s_A_L_I_N_A_N _ _,] WALIKOTATARAKAN PROVINS! KALIMANTANUTARA PERATURAN DAERAH KOTA TARAKAN NOMOR 3 TAHUN 2019 TENTANG HARIJADIKOTATARAKAN DENGAN RAHMATTUHAN YANG MAHA ESA WALIKOTATARAKAN , Menimbang: a. bahwa dengan ditetapkannya Kota Tarakan sebagai Daerah Otonom sehingga perlu melestarikan nilai• nilai sejarah daerah yang hidup dan berkembang dalam Negara Kesatuan Republik Indonesia; b. bahwa hari jadi Kota Tarakan merupakan tonggak sejarah penting bagi masyarakat Kota Tarakan untuk memperkokoh jati diri sekaligus untuk meningkatkan motivasi, rasa kecintaan, kebanggaan dan rasa memiliki guna mewujudkan rasa cinta tanah air dengan memperingati Hari Jadi Kota Tarakan; c. bahwa berdasarkan pertimbangan sebagaimana dimaksud pada huruf a dan huruf b, perlu menetapkan Peraturan Daerah tentang Hari Jadi Kota Tarakan; Mengingat: 1. Pasal 18 ayat ( 6) Undang-Undang Dasar Republik Indonesia Tahun 1945; 2. Undang-Undang Nomor 29 Tahun 1997 tentang Pembentukan Kotamadya Daerah Tingkat II Tarakan (Lembaran Negara Republik Indonesia Tahun 1997 Nomor 82, Tambahan Lembaran Negara Republik Indonesia Nomor 3711); 3. Undang-Undang Nomor 20 Tahun 2012 tentang Pembentukan Provinsi Kalimantan Utara (Lembaran Negara Republik Indonesia Tahun 2012 Nomor 229, Tambahan Lembaran Negara Republik Indonesia Nomor 5362); --------------~~~ ~ 2 4. Undang-Undang Nomor 23 Tahun 2014 tentang Pemerintahan Daerah (Lembaran Negara Republik Indonesia Tahun 2014 Nomor 244, Tambahan Lembaran Negara Republik Indonesia Nomor 5587) sebagaimana telah diubah beberapa kali terakhir dengan Undang-Undang Nomor 9 Tahun 2015 tentang Perubahan Kedua Atas Undang-Undang Nomor 23 Tahun 2014 tentang Pemerintahan Daerah (Lembaran Negara Republik Indonesia Tahun 2015 Nomor 58, Tambahan Lembaran Negara Republik Indonesia Nomor 5679); Dengan Persetujuan Bersama DEWAN PERWAKILANRAKYAT DAERAH KOTA TARAKAN dan WALIKOTATARAKAN Menetapkan PERATURAN DAERAH TENTANG HARI JADI KOTA TARAKAN. -

PT. PANCA MITRA MULTIPERDANA Tbk. Office : Jl
PT. PANCA MITRA MULTIPERDANA Tbk. Office : Jl. Bubutan 16-22 Kav. A No.1-3 Surabaya 60174 Indonesia Ph. 62 31 5459213, 5462539 Fax. 62 31 5459161 Email : [email protected] Situbondo Plant 1 : Jl. Raya Banyuwangi Km.10 Situbondo Indonesia Situbondo Plant 2 : Jl. Raya Wonokoyo No.3 Dusun Lauk Bindung RT.02/RW.03 Situbondo Indonesia Tarakan Factory : Jl. Kurau RT.16 Juata Laut, Tarakan North Kalimantan Indonesia Surabaya, 2 Juli 2021 Nomor: 030/PMMP/CORSEC/VII/2021 Kepada Yth. Otoritas Jasa Keuangan Gedung Sumitro Djojohadikusumo, Jl. Lapangan Banteng Timur No.2-4 Jakarta 10710 UP : Kepala Eksekutif Pengawas Pasar Modal Otoritas Jasa Keuangan Bukti Iklan Pemanggilan Rapat Umum Pemegang Saham Tahunan dan Perihal : Luar Biasa PT Panca Mitra Multiperdana Tbk (“Perseroan”) Lampiran : 1 set Dengan hormat, Merujuk kepada POJK No. 15/POJK.04/2020 tentang Rencana dan Penyelenggaraan Rapat Umum Pemegang Saham Perusahaan Terbuka dan sehubungan dengan rencana penyelenggaraan Rapat Umum Pemegang Saham Tahunan dan Luar Biasa (“Rapat”) PT Panca Mitra Multiperdana Tbk yang akan dilaksanakan pada tanggal 26 Juli 2021, dengan ini kami sampaikan bahwa pengumuman Rapat telah kami iklankan dalam situs web Kustodian Sentral Efek lndonesia (KSEI), situs web Bursa Efek lndonesia (BEl) dan situs web Perseroan pada tanggal 2 Juli 2021. Terlampir kami sampaikan bukti iklan pengumuman Rapat yang telah dimuat dalam situs web Kustodian Sentral Efek lndonesia (KSEI), situs web Bursa Efek lndonesia (BEl) dan situs web Perseroan pada tanggal 2 Juli 2021. Demikian informasi ini kami sampaikan. Atas perhatiannya, kami ucapkan terima kasih. Hormat Kami, PT Panca Mitra Multiperdana Tbk Martinus Soesilo Direktur Utama PT. -

Summary Audit Report
SUMMARY AUDIT REPORT Name of Cyanide Transportation Facility: PT Trans Continent Name of Facility Owner: Mr Ismail Rasyid Name of Responsible Manager: Mr Ismail Rasyid Address: Jl. Tebet Raya No. 22A, Tebet Barat State/Province: South Jakarta 12810 Country: Indonesia Telephone: +62 21 8378 7104 E-Mail: [email protected] Location detail and description of operation: Overview PTTC Inbound shipments of cyanide containers consists of two supply chains for two mines sites as follows: 1. PT J Resources Bolaang Mongondow Bakan (JRBM) PTTC transportation supply chain starting from PT Terminal Petikemas Surabaya (TPS), thereafter these containers are being transported by Hacaca (Subcontractor) to Surabaya Tanjong Perak Domestic seaport; followed by loading onboard TANTO commercial vessel for sea transportation to Port of BITUNG. Upon arrival at Bitung; these containers will be stored at Dangerous Goods yard located within Port of Bitung, land transportation for delivery to JRBM in Bakan North Sulawesi by PTTC own transportation and drivers. 2. PT Sago Prima (JResources Group) SPP PTTC transportation supply chain starting from PT Terminal Petikemas Surabaya (TPS), thereafter these containers are being transported by Hacaca (Subcontractor) to Surabaya Tanjong Perak Domestic seaport; followed by loading onboard Salam Pacific International Line (SPIL) commercial vessel for sea transportation to Port of Tarakan; these containers will be stored at SPIL container yard located within Port of Tarakan, outsourced LCT/Barge along Seruyung River, operated by PT. Lautan Jaya Utama and land transportation from Seruyung Jetty to mine site (Northern Kalimantan) by PTTC own transportation and drivers. Name of Facility: PTTC Signature of Lead Auditor Date 3rd Jan 2018 & Technical Expert SUMMARY AUDIT REPORT Auditor’s Finding This operation is in full compliance ☐☐☐ in substantial compliance *(see below) ☐☐☐ not in compliance with the International Cyanide Management Code. -

Kajian Meteorologi Dan Oseanografi Perairan Balikpapan-Tarakan Sebagai Rute Health Boat Untuk Pelayanan Kesehatan Pulau Terpencil Kalimantan
SPECTA Journal of Technology, Vol. 1, No. 1, March 2017 ISSN : 2549-2713 Kajian Meteorologi dan Oseanografi Perairan Balikpapan-Tarakan sebagai Rute Health Boat untuk Pelayanan Kesehatan Pulau Terpencil Kalimantan Luh Putri Adnyani Teknik Perkapalan , Institut Teknologi Kalimantan, Balikpapan. Email: [email protected] Abstract Distribution of medical facilities in Indonesia is uneven, so residents in remote islands have difficulties to reach it. We need to build health boat, a floating facility that provide standard services like in the hospital, especially in Derawan and Tarakan Islands in East Kalimantan. This Health boat has a homebase in Balikpapan. Therefore, the condition of the sea between Balikpapan, Derawan, and Tarakan need to be reviewed. One method to determine the condition of the sea is analysis of meteorological and oceanographic, which enable us to get the maximum wave height estimation. Long tern wave forecast can be obtained by using statistical methods of measurement to provide data of high wave in 1, 10, 25, 50 and 100 years. Keywords: Health Boat, Meteorology, Oceanografy, Wave Forecasting Abstrak Persebaran fasilitas medis di Indonesia tidak merata, sehingga warga di pelosok dan kepulauan sulit menjangkaunya. Perlu dibangun health boat, fasilitas terapung yang menyediakan layanan standar rumah sakit. Terutama yang beroperasi di wilayah Kepulauan Derawan dan Tarakan di Kalimantan. Health Boat ini memiliki homebase di Balikpapan, sehingga kondisi perairan dari Balikpapan sampai ke Derawan dan Tarakan perlu ditinjau. Salah satu metode untuk mengetahui kondisi perairan adalah analisa meteorology dan oceanografi sehingga mendapatkan perkiraan tinggi gelombang maksimal. Ramalan gelombang jangka waktu panjang dapat diperoleh dengan metode statistik dari data pengukuran yang tersedia dan mendapatkan tinggi gelombang 1, 10, 25, 50 dan 100 tahun. -
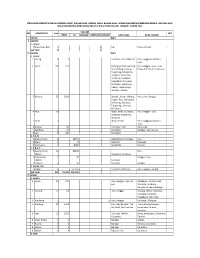
Data Operasional
KEGIATAN DOMESTIK MASUK HEWAN HIDUP, BAHAN ASAL HEWAN, HASIL BAHAN ASAL HEWAN DAN MEDIA PEMBAWA/BENDA LAIN MELALUI BALAI KARANTINA PERTANIAN KELAS II PALU PADA BULAN JANUARI TAHUN 2020 VOLUME NO KOMODITAS Frek KET EKOR Kg LEMBAR KEMASAN KOLONI KOTA ASAL KOTA TUJUAN I IMPOR NIHIL II EKSPOR a. Hewan 1 Kupu Kupu Mati 2 4 Sigi Tokyo, Jepang Sub Total 2 4 III DOMAS NIHIL a. Hewan 1 Anjing 8 9 Surabaya, Batu, Makassar Palu, Banggai Kepulauan, Luwu 2 Ayam 101 225 Badung, Bantul, Buleleng, Palu, Banggai, Luwu, Luwu Deli Serdang, Sidoarjo, Timur, Toli toli, Tojo Una una Tangerang, Balikpapan, Denpasar, Makassar, Semarang, Surabaya, Yogyakarta, Denpasar, Gorontalo, Kepulauan Talaud, Kotamobogu, Manado, Tarakan 3 Burung 35 2042 Boyolali, Klaten, Malang, Palu, Luwu, Banggai Sragen, Palu, Denpasara, Semarang, Surabaya, Tangerang , Sidoarjo, Gorontalo 4 DOC 30 20500 Bogor, Kediri, Surabaya, Palu, Banggai, Luwu Jombang, Mojokerto, Makassar 5 DOQ 3 6400 Blitar, Sleman Palu, Banggai Kepulauan, Luwu 6 Kucing 4 20 Surabaya , Batu Palu, Luwu 7 Kambing 2 29 Gorontalo Banggai, Tojo Una una 8 sapi 6 100 Gorontalo b. B.A.H 1 Daging Ayam 6 30015 Jakarta Barat, Surabaya Palu 2 Madu 1 8 Mataram Donggala 3 Ice Cream 1 8000 Mojokerto Toli toli c. H.B.A.H 1 Daging Ayam 10 82200 Palu Olahan Mojokerto, Surabaya 2 Daging Sapi 2 30 Banggai, Luwu Olahan Surabaya 3 Yoghurt 4 90 Surabaya Banggai d. Benda Lain 1 Pakan 5 371250 Surabaya, Makassar Palu, Banggai, Toli toli Sub Total 218 29,325 491,593 IV DOKEL a. Hewan 1 Ayam 64 140 Palu, Banggai, Tojo Una Balikpapan, Jakarta -

PENGADILAN Ml LITER I - 07 BALIKPAPAN LAMPIRAN Surat Kadilmil 1-07 Balikpapan JL
PENGADILAN Ml LITER I - 07 BALIKPAPAN LAMPIRAN Surat Kadilmil 1-07 Balikpapan JL. SYARIFUDDIN YOES NO. 39 BALIKPAPAN Nomor : W1-MIL07/B-281/HK.0WIII/2019 E-mail: [email protected] Tanggal: &O Agustus2019 Telp. (0542) 8520025, (0542) 8520026, Fax: (0542) 8520024 Daftar Papera dan Ankum Wilayah Hukum Dilmil I-07 Balikpapan Balikpapan e2 Agustus 2019 1.Pangdam Vl/Mulawarman W1-MIL07/280 /KP.01. Will/2019 Nomor 2.Danrem 091/Antasri Kasifikasi Biasa 3.Danlantamal Xlll/Tarakan 4.Danlanud Dhomber Balikpapan Lampiran 1(satu) lembar 5.Danlanud Anang Busra Tarakan Perihal Sosialisasi informasi dan pengaduan Pengadilan Militer I-07 Balikpapan 6.Danbrigif 24/Bulungan Cakti Kepada 7.Danpomdam Vl/Mulawarman 8.Kababinminvetcatdam Vl/Mulawarman Yth. Papera, Ankum di Wilayah 9.Kazidam Vl/Mulawarman Hukum Pengadilan Militer 10.Kahubdam Vl/Mulawarman 11.Kapaldam Vl/Mulawarman I-07 Balikpapan 12.Kabekang Vl/Mulawarman 13.Kakesdam Vl/Mulawarman di 14.Karumkit Vl/Mulawarman 15.Kakudam Vl/Mulawarman Tempat. 16.Kakumdam Vl/Mulawarman 17.Kaajendam Vl/Mulawarman 1.Dalam rangka pelaksanaan tata pemerintahan yang baik (good governance) 18.Kabintaldam Vl/Mulawarman keterbukaan informasi serta layanan yang bebas dari korupsi, kolusi dan nepotisme 19.Kajasdam Vl/Mulawarman serta Pembangunan Zona Integritas Menuju Wilayah Bebas Korupsi (WBK) dan 20.Katopdam Vl/Mulawarman Wilayah Birokrasi Bersih dan Melayani (WBBM) di Lingkungan Instansi Pengadilan 21.Kapendan Vl/Mulawarman 22.Kainfolahtadam Vl/Mulawarman Militer I-07 Balikpapan. 23.Kasetumdam Vl/Mulawarman 24.Kasandidam Vl/Mulawarman 2.Sehubungan hal tersebut di atas, bahwa layanan informasi dan pengaduan 25.Dandenmadam Vl/Mulawarman Pengadilan Militer I-07 Balikpapan dapat disampaikan kepada kami melalui sarana 26.Dandeninteldam Vl/Mulawarman www.siwas.