A Molecular Phylogeny of the Palaearctic and O.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Scope: Munis Entomology & Zoology Publishes a Wide Variety of Papers
732 _____________Mun. Ent. Zool. Vol. 7, No. 2, June 2012__________ STRUCTURE OF LEPIDOPTEROCENOSES ON OAKS QUERCUS DALECHAMPII AND Q. CERRIS IN CENTRAL EUROPE AND ESTIMATION OF THE MOST IMPORTANT SPECIES Miroslav Kulfan* * Department of Ecology, Faculty of Natural Sciences, Comenius University, Mlynská dolina B-1, SK-84215 Bratislava, SLOVAKIA. E-mail: [email protected] [Kulfan, M. 2012. Structure of lepidopterocenoses on oaks Quercus dalechampii and Q. cerris in Central Europe and estimation of the most important species. Munis Entomology & Zoology, 7 (2): 732-741] ABSTRACT: On the basis of lepidopterous larvae a total of 96 species on Quercus dalechampii and 58 species on Q. cerris were recorded in 10 study plots of Malé Karpaty and Trnavská pahorkatina hills. The families Geometridae, Noctuidae and Tortricidae encompassed the highest number of found species. The most recorded species belonged to the trophic group of generalists. On the basis of total abundance of lepidopterous larvae found on Q. dalechampii from all the study plots the most abundant species was evidently Operophtera brumata. The most abundant species on Q. cerris was Cyclophora ruficiliaria. Based on estimated oak leaf area consumed by a larva it is shown that Lymantria dispar was the most important leaf-chewing species of both Q. dalechampii and Q. cerris. KEY WORDS: Slovakia, Quercus dalechampii, Q. cerris, the most important species. About 300 Lepidoptera species are known to damage the assimilation tissue of oaks in Central Europe (Patočka, 1954, 1980; Patočka et al.1999; Reiprich, 2001). Lepidoptera larvae are shown to be the most important group of oak defoliators (Patočka et al., 1962, 1999). -

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Thesis.Pdf (3.979Mb)
FACULTY OF BIOSCIENCES, FISHERIES AND ECONOMICS DEPARTMENT OF ARCTIC AND MARINE BIOLOGY Cyclically outbreaking geometrid moths in sub-arctic mountain birch forest: the organization and impacts of their interactions with animal communities — Ole Petter Laksforsmo Vindstad A dissertation for the degree of Philosophiae Doctor – October 2014 Cyclically outbreaking geometrid moths in sub-arctic mountain birch forest: the organization and impacts of their interactions with animal communities Ole Petter Laksforsmo Vindstad A dissertation for the degree of Philosophiae Doctor University of Tromsø – The arctic university of Norway Faculty of Biosciences, Fisheries and Economics Department of Arctic and Marine Biology Autumn 2014 1 Dedicated to everyone who has helped me along the way 2 Supervisors Professor Rolf Anker Ims1 Senior researcher Jane Uhd Jepsen2 1 Department of Arctic and Marine Biology, University of Tromsø, Tromsø, Norway 2 Norwegian Institute for Nature Research, Fram Centre, Tromsø, Norway Cover photos Front cover – Larvae of Epirrita autumnata feeding on mountain birch during a moth outbreak in northern Norway. Photo: Moritz Klinghardt Study I – Portrait of Agrypon flaveolatum. One of the most important larval parasitoid species in study I. Photo: Ole Petter Laksforsmo Vindstad Study II – Carcass of an Operophtera brumata larva, standing over the cocoon of its killer, the parasitoid group Protapanteles anchisiades/P. immunis/Cotesia salebrosa. Photo: Ole Petter Laksforsmo Vindstad Study III – Larva of the parasitoid group Phobocampe sp./Sinophorus crassifemur emerging from Agriopis aurantiaria host larva. Photo: Tino Schott Study IV – An area of healthy mountain birch forest, representative for the undamaged sampling sites in study IV and V. Photo: Jakob Iglhaut Study V – An area of mountain birch forest that has been heavily damaged by a moth outbreak, representative for the damaged sampling sites in study IV and V. -

Classical Biological Control of Arthropods in Australia
Classical Biological Contents Control of Arthropods Arthropod index in Australia General index List of targets D.F. Waterhouse D.P.A. Sands CSIRo Entomology Australian Centre for International Agricultural Research Canberra 2001 Back Forward Contents Arthropod index General index List of targets The Australian Centre for International Agricultural Research (ACIAR) was established in June 1982 by an Act of the Australian Parliament. Its primary mandate is to help identify agricultural problems in developing countries and to commission collaborative research between Australian and developing country researchers in fields where Australia has special competence. Where trade names are used this constitutes neither endorsement of nor discrimination against any product by the Centre. ACIAR MONOGRAPH SERIES This peer-reviewed series contains the results of original research supported by ACIAR, or material deemed relevant to ACIAR’s research objectives. The series is distributed internationally, with an emphasis on the Third World. © Australian Centre for International Agricultural Research, GPO Box 1571, Canberra ACT 2601, Australia Waterhouse, D.F. and Sands, D.P.A. 2001. Classical biological control of arthropods in Australia. ACIAR Monograph No. 77, 560 pages. ISBN 0 642 45709 3 (print) ISBN 0 642 45710 7 (electronic) Published in association with CSIRO Entomology (Canberra) and CSIRO Publishing (Melbourne) Scientific editing by Dr Mary Webb, Arawang Editorial, Canberra Design and typesetting by ClarusDesign, Canberra Printed by Brown Prior Anderson, Melbourne Cover: An ichneumonid parasitoid Megarhyssa nortoni ovipositing on a larva of sirex wood wasp, Sirex noctilio. Back Forward Contents Arthropod index General index Foreword List of targets WHEN THE CSIR Division of Economic Entomology, now Commonwealth Scientific and Industrial Research Organisation (CSIRO) Entomology, was established in 1928, classical biological control was given as one of its core activities. -

The Barbastelle in Bovey Valley Woods
The Barbastelle in Bovey Valley Woods A report prepared for The Woodland Trust The Barbastelle in Bovey Valley Woods Andrew Carr, Dr Matt Zeale & Professor Gareth Jones School of Biological Sciences, University of Bristol, Life Sciences Building, 24 Tyndall Avenue, Bristol, BS8 1TQ Report prepared for The Woodland Trust October 2016 Acknowledgements Thanks to: Dave Rickwood of the Woodland Trust for his central role and continued support throughout this project; Dr Andrew Weatherall of the University of Cumbria; Simon Lee of Natural England and James Mason of the Woodland Trust for helpful advice; Dr Beth Clare of Queen Mary University of London for support with molecular work; the many Woodland Trust volunteers and assistants that provided their time to the project. We would particularly like to thank Tom ‘the tracker’ Williams and Mike ‘the trapper’ Treble for dedicating so much of their time. We thank the Woodland Trust, Natural England and the Heritage Lottery Fund for funding this research. We also appreciate assistance from the local landowners who provided access to land. i Contents Acknowledgements i Contents ii List of figures and tables iii 1 Introduction 1 1.1 Background 1 1.2 The Barbastelle in Bovey Valley Woods 2 1.3 Objectives 2 2 Methods 2 2.1 Study area 2 2.2 Bat capture, tagging and radio-tracking 3 2.3 Habitat mapping 4 2.4 Analysis of roost preferences 5 2.5 Analysis of ranges and foraging areas 7 2.6 Analysis of diet 7 3 Results 8 3.1 Capture data 8 3.2 Roost selection and preferences 9 3.3 Ranging and foraging 14 3.4 Diet 17 4 Discussion 21 4.1 Roost use 21 4.2 Ranging behaviour 24 4.3 Diet 25 5 Conclusion 26 References 27 Appendix 1 Summary table of all bat captures 30 Appendix 2 Comparison of individual B. -

(Prout)(Geometridae
The LepidopterologicalSocietyLepidopterological Society of Japan eetva Z'ans. tepid. Soc. Jopan 59 (2): 171-185,March 2008 Notes onAlcis variegata (Moore),A. colorijlera (Prout) (Geometridae, Ennominae), and their allies from the Sunda Islands, with descriptions of two new species Rikio SATo 2-27-29, Shindori-nishi, Nishi-ku, Niigata, 950-2036 Japan Abstract Two species-groups ofAlcis from the Sunda Islands are revised. The A. variegata cum- plex: A, variegata (Moore), A. com,ariata (Prout), stat. & comb. nov,, A. hemiphanes (ProuO, A. cockaynei (Prout), A. Iuizi sp. nov. (Sumatra), A. paukstadti sp. nov, (Flores>, A. praevariegata (ProuO. Alcis taiwanovariegata nom. nov. is proposed for Boarmia suhochrearia Wileman & South, 1917, currently assigned to Alcis and preeccupied by Leech, IS97, The A. colorCfera com- plex: A. coloritlera (Prout), A. periphracta (Prout). Key words Geumetridae, Ennominae, Atcis variegata, Alcis color(fera, taxonomic notes, new species, Sunda Islands. In my previous paper (Sato, 2005), two species groups of the genus Atcis Cunis from Southeast Asia, the pammicra and maculata cQmplexes, were revised. In this paper, I will give a taxonomic account of two further species groups of Alcis from the Sunda Islands (Borneo, Sumatra, Java, Flores) in Southeast Asia, with descriptions of two new species, A, "variegata variegata andA. color(feva, and species allied to them will be named here the L`colorijleia complex" and complex" without strict definition, for the sake of convenience, No species belonging to the two complexes have been found from Sulawesi. For precise identification of the taxonomically more confusing species, I examined all available type specimens and their genitalia at the Natura] History Museum, London, UK, when I visited there in 2002. -

Macrolepidoptera Inventory of the Chilcotin District
Macrolepidoptera Inventory of the Chilcotin District Aud I. Fischer – Biologist Jon H. Shepard - Research Scientist and Crispin S. Guppy – Research Scientist January 31, 2000 2 Abstract This study was undertaken to learn more of the distribution, status and habitat requirements of B.C. macrolepidoptera (butterflies and the larger moths), the group of insects given the highest priority by the BC Environment Conservation Center. The study was conducted in the Chilcotin District near Williams Lake and Riske Creek in central B.C. The study area contains a wide variety of habitats, including rare habitat types that elsewhere occur only in the Lillooet-Lytton area of the Fraser Canyon and, in some cases, the Southern Interior. Specimens were collected with light traps and by aerial net. A total of 538 species of macrolepidoptera were identified during the two years of the project, which is 96% of the estimated total number of species in the study area. There were 29,689 specimens collected, and 9,988 records of the number of specimens of each species captured on each date at each sample site. A list of the species recorded from the Chilcotin is provided, with a summary of provincial and global distributions. The habitats, at site series level as TEM mapped, are provided for each sample. A subset of the data was provided to the Ministry of Forests (Research Section, Williams Lake) for use in a Flamulated Owl study. A voucher collection of 2,526 moth and butterfly specimens was deposited in the Royal BC Museum. There were 25 species that are rare in BC, with most known only from the Riske Creek area. -

Systematic Account
Systematic Account Subfamily Ennominae DUpONChEL, 1845 (continued from volume 5) Abraxini + Cassymini + Eutoeini group of tribes (part): Addition (other species treated in volume 5) The tribes Abraxini, Cassymini and Eutoeini are morphologically similar (see SKOU & SIh VONEN 2015 for details) and their close relationship is supported by molecular data also (SIhVONEN et al. 2011). An extensive molecular study on the Geometroidea phylogeny is under preparation and preliminary results continue to support the relationships of the three mentioned Ennominae groups including the genus Odontognophos WEhrLI, 1951 (M UrILLO-RAMOS et al. 2019) which is tentatively placed here in the tribe Abraxini. Morphol- ogy of the genus Dicrognophos WEhrLI, 1951 also supports association with the mentioned group, tribe Cassymini (it has thus far not been included in a molecular study). Both genera are transferred here from the Gnophini (e.g. HAUSMANN et al. 2004; 2011a) to the Abraxini + Cassymini + Eutoeini group. Odontognophos had been included in the broad concept of Macariini in BELJAEV (2016). In volume 5 of the Geometrid Moths of Europe (SKOU & SIhVONEN 2015) the following genera of the mentioned tribal group were treated: Abraxas LEACh, 1815, Ligdia GUENÉE, 1858, Lomaspilis HÜBNEr, 1825 and Stegania GUENÉE, 1845. The group is diagnosed by the following characters (HOLLOwAY 1994; SKOU & SIhVONEN 2015): Valva divided, its dorsal arm often narrow and curved, setose apically. Male 8th sternite unmodified. Fovea in male forewing often present. Chaetosemata present. Trans- verse setal comb often present on male 3rd abdominal sternite. Forewing radial veins often reduced in number. Female structures variable, diagnostic characters not yet identified. -
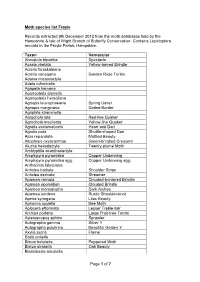
Page 1 of 7 Moth Species List Froyle Records
Moth species list Froyle Records extracted 9th December 2012 from the moth databases held by the Hampshire & Isle of Wight Branch of Butterfly Conservation. Contains Lepidoptera records in the Froyle Parish, Hampshire. Taxon Vernacular Abrostola tripartita Spectacle Acasis viretata Yellow-barred Brindle Acleris forsskaleana Acleris variegana Garden Rose Tortrix Adaina microdactyla Adela rufimitrella Agapeta hamana Agonopterix arenella Agonopterix heracliana Agriopis leucophaearia Spring Usher Agriopis marginaria Dotted Border Agriphila straminella Agrochola lota Red-line Quaker Agrochola macilenta Yellow-line Quaker Agrotis exclamationis Heart and Dart Agrotis puta Shuttle-shaped Dart Alcis repandata Mottled Beauty Allophyes oxyacanthae Green-brindled Crescent Alucita hexadactyla Twenty-plume Moth Amblyptilia acanthadactyla Amphipyra pyramidea Copper Underwing Amphipyra pyramidea agg. Copper Underwing agg. Anthophila fabriciana Anticlea badiata Shoulder Stripe Anticlea derivata Streamer Apamea crenata Clouded-bordered Brindle Apamea epomidion Clouded Brindle Apamea monoglypha Dark Arches Apamea sordens Rustic Shoulder-knot Apeira syringaria Lilac Beauty Aphomia sociella Bee Moth Aplocera efformata Lesser Treble-bar Archips podana Large Fruit-tree Tortrix Asteroscopus sphinx Sprawler Autographa gamma Silver Y Autographa pulchrina Beautiful Golden Y Axylia putris Flame Batia unitella Biston betularia Peppered Moth Biston strataria Oak Beauty Blastobasis adustella Page 1 of 7 Blastobasis lacticolella Cabera exanthemata Common Wave Cabera -

2004 IUFRO Forest Genetics Meeting Proceedings 1
2004 IUFRO Forest Genetics Meeting Proceedings 1 2004 IUFRO Forest Genetics Meeting Proceedings 2 2004 IUFRO Forest Genetics Meeting Proceedings Table of Contents Foreword_______________________________________________________ 4 Table of Contents – Oral Presentations____________________________ 6 Oral Presentations _______________________________________________ 17 Table of Contents – Poster Presentations___________________________ 400 Poster Presentations _____________________________________________ 403 Participant List _________________________________________________ 467 Title Index ____________________________________________________ 479 Speaker Index __________________________________________________ 485 3 2004 IUFRO Forest Genetics Meeting Proceedings FOREWORD In November 2004, North Carolina State University hosted a joint conference of multiple working parties related to breeding and genetic resource management of IUFRO Division 2. The papers and abstracts that follow in this proceeding were presented at this conference entitled "Forest Genetics and Tree Breeding in the Age of Genomics - Progress and Future". This international conference brought together geneticists, breeders, applied and basic scientists, managers and professional foresters to exchange the latest information on forest genetics and tree breeding, with special focus on potential application of biotechnology and genomics in the future. Given that the topics were important, timely, and pertinent to scientists worldwide, a total of 231 people from 22 countries participated -

Climatic Condition in Mandi Distric
Research Paper Volume : 4 | Issue : 1 | January 2015 • ISSN No 2277 - 8179 Taxonomic Studies on Light Trap Collected Biotechnology Species of Abraxas Leach Under Agro- KEYWORDS : Taxonomic, Genitalia, Wing, Adeagus Climatic Condition in Mandi District of Himachal Pradesh Forest Protection Division, Himalayan Forest Research Institute, Conifer Campus VIKRANT THAKUR Panthaghati, Shimla Himachal Pradesh 171009 MANOJ KUMAR Faculty of Biotechnology, Shoolini university, Solan HP-172230 ABSTRACT The light trap collection by using mercury lamp indicated that Abraxas Leach under agro-climatic condition of Mandi district of Himachal Pradesh is comprises of three species viz. Abraxas picaria Moore, Abraxas leucostola Hampson and Abraxas sylvata Scopoli. These species obatain their peak in July- August . Abraxas leucostola Hampson was found most abundant followed by Abraxas picaria Moore and Abraxas sylvata Scopoli on the basis of daily catches. The taxonomic study on these spe- cies carried out and described in detail. A key to the species of Abraxas Leach has also been provided for their easy identification. Introduction: DEX (Beccaloni et.al. 2003). The hierarchy of different moth is This moth is mostly white with brownish patches across all of given by Van Nieukerken et al. (2011) the wings. There are small areas of pale gray on the forewings and hind wings. They resemble bird droppings while resting on Result: the upper surface of leaves. The adults fly from late May to early Abraxas picaria Moore August. They are attracted to light. The wingspan is 38 mm. to picariaMoore, 1868, Proc. zool. Soc. Lond. 1893(2): 393 (Abraxas). 48 mm. The moth is nocturnal and is easy to find during the day. -

Bosco Palazzi
SHILAP Revista de Lepidopterología ISSN: 0300-5267 ISSN: 2340-4078 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Bella, S; Parenzan, P.; Russo, P. Diversity of the Macrolepidoptera from a “Bosco Palazzi” area in a woodland of Quercus trojana Webb., in southeastern Murgia (Apulia region, Italy) (Insecta: Lepidoptera) SHILAP Revista de Lepidopterología, vol. 46, no. 182, 2018, April-June, pp. 315-345 Sociedad Hispano-Luso-Americana de Lepidopterología España Available in: https://www.redalyc.org/articulo.oa?id=45559600012 How to cite Complete issue Scientific Information System Redalyc More information about this article Network of Scientific Journals from Latin America and the Caribbean, Spain and Journal's webpage in redalyc.org Portugal Project academic non-profit, developed under the open access initiative SHILAP Revta. lepid., 46 (182) junio 2018: 315-345 eISSN: 2340-4078 ISSN: 0300-5267 Diversity of the Macrolepidoptera from a “Bosco Palazzi” area in a woodland of Quercus trojana Webb., in southeastern Murgia (Apulia region, Italy) (Insecta: Lepidoptera) S. Bella, P. Parenzan & P. Russo Abstract This study summarises the known records of the Macrolepidoptera species of the “Bosco Palazzi” area near the municipality of Putignano (Apulia region) in the Murgia mountains in southern Italy. The list of species is based on historical bibliographic data along with new material collected by other entomologists in the last few decades. A total of 207 species belonging to the families Cossidae (3 species), Drepanidae (4 species), Lasiocampidae (7 species), Limacodidae (1 species), Saturniidae (2 species), Sphingidae (5 species), Brahmaeidae (1 species), Geometridae (55 species), Notodontidae (5 species), Nolidae (3 species), Euteliidae (1 species), Noctuidae (96 species), and Erebidae (24 species) were identified.