In South Australia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cylindroiulus Truncorum (Silvestri): a New Milliped for Virginia (USA), with Natural History Observations (Julida: Julidae)
Banisteria, Number 20, 2002 © 2002 by the Virginia Natural History Society Cylindroiulus truncorum (Silvestri): A New Milliped for Virginia (USA), with Natural History Observations (Julida: Julidae) Jorge A. Santiago-Blay Department of Paleobiology, MRC-121 National Museum of Natural History 10th and Constitution Avenue Smithsonian Institution P.O. Box 37012 Washington, DC 20013-7012 Richard L. Hoffman Virginia Museum of Natural History Martinsville, Virginia 24112 Joseph B. Lambert and Yuyang Wu Department of Chemistry Northwestern University 2145 Sheridan Road Evanston, Illinois 60208-3113 INTRODUCTION truncorum for Raleigh, North Carolina, about 320 km SSE of Salem (Shelley, 1978) is the southernmost In the fall 2000, author SB cleared the underbrush known occurrence of this species in the United States. of an Eastern White Pine (Pinus strobus L.) grove in his This milliped has also been documented for Brazil backyard located in an urban area of Salem, Virginia (Chamberlin & Hoffman, 1958; Hoffman, 1999). (USA) by cutting and removing the lower branches. About a year later, he revisited the same trees and Natural History Observations noticed copious resinous exudations originating from the branch stumps, particularly on five of the trees. Berlese extractions from P. strobus leaf litter were There, he observed about twenty millipeds, later conducted in November 2001 and yielded a maximum identified as Cylindroiulus truncorum (Silvestri, 1896; of about 50 C. truncorum per 0.25 m2 (= 200 C. species group reviewed by Korsós & Enghoff, 1990), truncorum per m2). In his many years of studying soil attached to the resin, 1-2 meters above ground (Fig. 1). invertebrates and running numerous Berlese samples, Voucher specimens of Cylindroiulus truncorum are particularly in southwestern Virginia, RLH has seldom deposited at the Virginia Museum of Natural History encountered millipeds under pine litter. -

Millipedes (Diplopoda) from Caves of Portugal
A.S.P.S. Reboleira and H. Enghoff – Millipedes (Diplopoda) from caves of Portugal. Journal of Cave and Karst Studies, v. 76, no. 1, p. 20–25. DOI: 10.4311/2013LSC0113 MILLIPEDES (DIPLOPODA) FROM CAVES OF PORTUGAL ANA SOFIA P.S. REBOLEIRA1 AND HENRIK ENGHOFF2 Abstract: Millipedes play an important role in the decomposition of organic matter in the subterranean environment. Despite the existence of several cave-adapted species of millipedes in adjacent geographic areas, their study has been largely ignored in Portugal. Over the last decade, intense fieldwork in caves of the mainland and the island of Madeira has provided new data about the distribution and diversity of millipedes. A review of millipedes from caves of Portugal is presented, listing fourteen species belonging to eight families, among which six species are considered troglobionts. The distribution of millipedes in caves of Portugal is discussed and compared with the troglobiont biodiversity in the overall Iberian Peninsula and the Macaronesian archipelagos. INTRODUCTION All specimens from mainland Portugal were collected by A.S.P.S. Reboleira, while collectors of Madeiran speci- Millipedes play an important role in the decomposition mens are identified in the text. Material is deposited in the of organic matter, and several species around the world following collections: Zoological Museum of University of have adapted to subterranean life, being found from cave Copenhagen, Department of Animal Biology, University of entrances to almost 2000 meters depth (Culver and Shear, La Laguna, Spain and in the collection of Sofia Reboleira, 2012; Golovatch and Kime, 2009; Sendra and Reboleira, Portugal. 2012). Although the millipede faunas of many European Species were classified according to their degree of countries are relatively well studied, this is not true of dependence on the subterranean environment, following Portugal. -

The Coume Ouarnède System, a Hotspot of Subterranean Biodiversity in Pyrenees (France)
diversity Article The Coume Ouarnède System, a Hotspot of Subterranean Biodiversity in Pyrenees (France) Arnaud Faille 1,* and Louis Deharveng 2 1 Department of Entomology, State Museum of Natural History, 70191 Stuttgart, Germany 2 Institut de Systématique, Évolution, Biodiversité (ISYEB), UMR7205, CNRS, Muséum National d’Histoire Naturelle, Sorbonne Université, EPHE, 75005 Paris, France; [email protected] * Correspondence: [email protected] Abstract: Located in Northern Pyrenees, in the Arbas massif, France, the system of the Coume Ouarnède, also known as Réseau Félix Trombe—Henne Morte, is the longest and the most complex cave system of France. The system, developed in massive Mesozoic limestone, has two distinct resur- gences. Despite relatively limited sampling, its subterranean fauna is rich, composed of a number of local endemics, terrestrial as well as aquatic, including two remarkable relictual species, Arbasus cae- cus (Simon, 1911) and Tritomurus falcifer Cassagnau, 1958. With 38 stygobiotic and troglobiotic species recorded so far, the Coume Ouarnède system is the second richest subterranean hotspot in France and the first one in Pyrenees. This species richness is, however, expected to increase because several taxonomic groups, like Ostracoda, as well as important subterranean habitats, like MSS (“Milieu Souterrain Superficiel”), have not been considered so far in inventories. Similar levels of subterranean biodiversity are expected to occur in less-sampled karsts of central and western Pyrenees. Keywords: troglobionts; stygobionts; cave fauna Citation: Faille, A.; Deharveng, L. The Coume Ouarnède System, a Hotspot of Subterranean Biodiversity in Pyrenees (France). Diversity 2021, 1. Introduction 13 , 419. https://doi.org/10.3390/ Stretching at the border between France and Spain, the Pyrenees are known as one d13090419 of the subterranean hotspots of the world [1]. -

Some Centipedes and Millipedes (Myriapoda) New to the Fauna of Belarus
Russian Entomol. J. 30(1): 106–108 © RUSSIAN ENTOMOLOGICAL JOURNAL, 2021 Some centipedes and millipedes (Myriapoda) new to the fauna of Belarus Íåêîòîðûå ãóáîíîãèå è äâóïàðíîíîãèå ìíîãîíîæêè (Myriapoda), íîâûå äëÿ ôàóíû Áåëàðóñè A.M. Ostrovsky À.Ì. Îñòðîâñêèé Gomel State Medical University, Lange str. 5, Gomel 246000, Republic of Belarus. E-mail: [email protected] Гомельский государственный медицинский университет, ул. Ланге 5, Гомель 246000, Республика Беларусь KEY WORDS: Geophilus flavus, Lithobius crassipes, Lithobius microps, Blaniulus guttulatus, faunistic records, Belarus КЛЮЧЕВЫЕ СЛОВА: Geophilus flavus, Lithobius crassipes, Lithobius microps, Blaniulus guttulatus, фаунистика, Беларусь ABSTRACT. The first records of three species of et Dobroruka, 1960 under G. flavus by Bonato and Minelli [2014] centipedes and one species of millipede from Belarus implies that there may be some previous records of G. flavus are provided. All records are clearly synathropic. from the former USSR, including Belarus, reported under the name of G. proximus C.L. Koch, 1847 [Zalesskaja et al., 1982]. РЕЗЮМЕ. Приведены сведения о фаунистичес- The distribution of G. flavus in European Russia has been summarized by Volkova [2016]. ких находках трёх новых видов губоногих и одного вида двупарноногих многоножек в Беларуси. Все ORDER LITHOBIOMORPHA находки явно синантропные. Family LITHOBIIDAE The myriapod fauna of Belarus is still poorly-known. Lithobius (Monotarsobius) crassipes C.L. Koch, According to various authors, 10–11 species of centi- 1862 pedes [Meleško, 1981; Maksimova, 2014; Ostrovsky, MATERIAL EXAMINED. 1 $, Republic of Belarus, Minsk, Kra- 2016, 2018] and 28–29 species of millipedes [Lokšina, sivyi lane, among household waste, 14.07.2019, leg. et det. A.M. 1964, 1969; Tarasevich, 1992; Maksimova, Khot’ko, Ostrovsky. -

Millipedes and Centipedes
MILLIPEDES AND CENTIPEDES Integrated Pest Management In and Around the Home sprouting seeds, seedlings, or straw- berries and other ripening fruits in contact with the ground. Sometimes individual millipedes wander from their moist living places into homes, but they usually die quickly because of the dry conditions and lack of food. Occasionally, large (size varies) (size varies) numbers of millipedes migrate, often uphill, as their food supply dwindles or their living places become either Figure 1. Millipede (left); Centipede (right). too wet or too dry. They may fall into swimming pools and drown. Millipedes and centipedes (Fig. 1) are pedes curl up. The three species When disturbed they do not bite, but often seen in and around gardens and found in California are the common some species exude a defensive liquid may be found wandering into homes. millipede, the bulb millipede, and the that can irritate skin or burn the eyes. Unlike insects, which have three greenhouse millipede. clearly defined body sections and Life Cycle three pairs of legs, they have numer- Millipedes may be confused with Adult millipedes overwinter in the ous body segments and numerous wireworms because of their similar soil. Eggs are laid in clutches beneath legs. Like insects, they belong to the shapes. Wireworms, however, are the soil surface. The young grow largest group in the animal kingdom, click beetle larvae, have only three gradually in size, adding segments the arthropods, which have jointed pairs of legs, and stay underneath the and legs as they mature. They mature bodies and legs and no backbone. soil surface. -

Millipedes and Centipedes? Millipedes and Centipedes Are Both Arthropods in the Subphylum Myriapoda Meaning Many Legs
A Teacher’s Resource Guide to Millipedes & Centipedes Compiled by Eric Gordon What are millipedes and centipedes? Millipedes and centipedes are both arthropods in the subphylum Myriapoda meaning many legs. Although related to insects or “bugs”, they are not actually insects, which generally have six legs. How can you tell the difference between millipedes and centipedes? Millipedes have two legs per body segment and are typically have a body shaped like a cylinder or rod. Centipedes have one leg per body segment and their bodies are often flat. Do millipedes really have a thousand legs? No. Millipedes do not have a thousand legs nor do all centipedes have a hundred legs despite their names. Most millipedes have from 40-400 legs with the maximum number of legs reaching 750. No centipede has exactly 100 legs (50 pairs) since centipedes always have an odd number of pairs of legs. Most centipedes have from 30- 50 legs with one order of centipedes (Geophilomorpha) always having much more legs reaching up to 350 legs. Why do millipedes and centipedes have so many legs? Millipedes and centipedes are metameric animals, meaning that their body is divided into segments most of which are completely identical. Metamerization is an important phenomenon in evolution and even humans have a remnant of former metamerization in the repeating spinal discs of our backbone. Insects are thought to have evolved from metameric animals after specializing body segments for specific functions such as the head for sensation and the thorax for locomotion. Millipedes and centipedes may be evolutionary relatives to the ancestor of insects and crustaceans. -

Some Aspects of the Ecology of Millipedes (Diplopoda) Thesis
Some Aspects of the Ecology of Millipedes (Diplopoda) Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Monica A. Farfan, B.S. Graduate Program in Evolution, Ecology, and Organismal Biology The Ohio State University 2010 Thesis Committee: Hans Klompen, Advisor John W. Wenzel Andrew Michel Copyright by Monica A. Farfan 2010 Abstract The focus of this thesis is the ecology of invasive millipedes (Diplopoda) in the family Julidae. This particular group of millipedes are thought to be introduced into North America from Europe and are now widely found in many urban, anthropogenic habitats in the U.S. Why are these animals such effective colonizers and why do they seem to be mostly present in anthropogenic habitats? In a review of the literature addressing the role of millipedes in nutrient cycling, the interactions of millipedes and communities of fungi and bacteria are discussed. The presence of millipedes stimulates fungal growth while fungal hyphae and bacteria positively effect feeding intensity and nutrient assimilation efficiency in millipedes. Millipedes may also utilize enzymes from these organisms. In a continuation of the study of the ecology of the family Julidae, a comparative study was completed on mites associated with millipedes in the family Julidae in eastern North America and the United Kingdom. The goals of this study were: 1. To establish what mites are present on these millipedes in North America 2. To see if this fauna is the same as in Europe 3. To examine host association patterns looking specifically for host or habitat specificity. -

Biodiversity from Caves and Other Subterranean Habitats of Georgia, USA
Kirk S. Zigler, Matthew L. Niemiller, Charles D.R. Stephen, Breanne N. Ayala, Marc A. Milne, Nicholas S. Gladstone, Annette S. Engel, John B. Jensen, Carlos D. Camp, James C. Ozier, and Alan Cressler. Biodiversity from caves and other subterranean habitats of Georgia, USA. Journal of Cave and Karst Studies, v. 82, no. 2, p. 125-167. DOI:10.4311/2019LSC0125 BIODIVERSITY FROM CAVES AND OTHER SUBTERRANEAN HABITATS OF GEORGIA, USA Kirk S. Zigler1C, Matthew L. Niemiller2, Charles D.R. Stephen3, Breanne N. Ayala1, Marc A. Milne4, Nicholas S. Gladstone5, Annette S. Engel6, John B. Jensen7, Carlos D. Camp8, James C. Ozier9, and Alan Cressler10 Abstract We provide an annotated checklist of species recorded from caves and other subterranean habitats in the state of Georgia, USA. We report 281 species (228 invertebrates and 53 vertebrates), including 51 troglobionts (cave-obligate species), from more than 150 sites (caves, springs, and wells). Endemism is high; of the troglobionts, 17 (33 % of those known from the state) are endemic to Georgia and seven (14 %) are known from a single cave. We identified three biogeographic clusters of troglobionts. Two clusters are located in the northwestern part of the state, west of Lookout Mountain in Lookout Valley and east of Lookout Mountain in the Valley and Ridge. In addition, there is a group of tro- globionts found only in the southwestern corner of the state and associated with the Upper Floridan Aquifer. At least two dozen potentially undescribed species have been collected from caves; clarifying the taxonomic status of these organisms would improve our understanding of cave biodiversity in the state. -
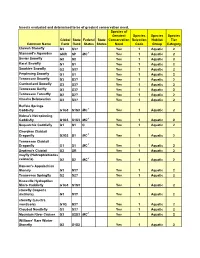
Insects Evaluated and Determined to Be of Greatest Conservation Need
Insects evaluated and determined to be of greatest conservation need. Species of Greatest Species Species Species Global State Federal State Conservation Selection Habitat Tier Common Name Rank Rank Status Status Need Code Group Category Etowah Stonefly G3 S3? Yes 1 Aquatic 2 Stannard's Agarodes GNR SP MC 1 Yes 1 Aquatic 2 Sevier Snowfly G2 S2 Yes 1 Aquatic 2 Karst Snowfly G1 S1 Yes 1 Aquatic 2 Smokies Snowfly G2 S3? Yes 1 Aquatic 2 Perplexing Snowfly G1 S1 Yes 1 Aquatic 2 Tennessee Snowfly G3 S3? Yes 1 Aquatic 2 Cumberland Snowfly G3 S3? Yes 1 Aquatic 2 Tennessee Sallfly G3 S3? Yes 1 Aquatic 2 Tennessee Forestfly G2 S2? Yes 1 Aquatic 2 Cheaha Beloneurian G3 S3? Yes 1 Aquatic 2 Buffalo Springs Caddisfly G1G3 S1S3 MC 1 Yes 1 Aquatic 2 Helma's Net-spinning Caddisfly G1G3 S1S3 MC 1 Yes 1 Aquatic 2 Sequatchie Caddisfly G1 S1 C Yes 1 Aquatic 2 Cherokee Clubtail Dragonfly G2G3 S1 MC 1 Yes 1 Aquatic 2 Tennessee Clubtail Dragonfly G1 S1 MC 1 Yes 1 Aquatic 2 Septima's Clubtail G2 SR Yes 1 Aquatic 2 mayfly (Habrophlebiodes celeteria) G2 S2 MC 1 Yes 1 Aquatic 2 Hanson's Appalachian Stonely G3 S3? Yes 1 Aquatic 2 Tennessee Springfly G2 S2? Yes 1 Aquatic 2 Knoxville Hydroptilan Micro Caddisfly G1G3 S1S3 Yes 1 Aquatic 2 stonefly (Isoperla distincta) G3 S3? Yes 1 Aquatic 2 stonefly (Leuctra monticola) G1Q S3? Yes 1 Aquatic 2 Clouded Needlefly G3 S3? Yes 1 Aquatic 2 Mountain River Cruiser G3 S2S3 MC 1 Yes 1 Aquatic 2 Williams' Rare Winter Stonefly G2 S1S2 Yes 1 Aquatic 2 Con't...Insects evaluated and determined to be of greatest conservation need. -

University of Copenhagen
Myriapods (Myriapoda). Chapter 7.2 Stoev, Pavel; Zapparoli, Marzio; Golovatch, Sergei; Enghoff, Henrik; Akkari, Nasrine; Barber, Anthony Published in: BioRisk DOI: 10.3897/biorisk.4.51 Publication date: 2010 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Stoev, P., Zapparoli, M., Golovatch, S., Enghoff, H., Akkari, N., & Barber, A. (2010). Myriapods (Myriapoda). Chapter 7.2. BioRisk, 4(1), 97-130. https://doi.org/10.3897/biorisk.4.51 Download date: 07. apr.. 2020 A peer-reviewed open-access journal BioRisk 4(1): 97–130 (2010) Myriapods (Myriapoda). Chapter 7.2 97 doi: 10.3897/biorisk.4.51 RESEARCH ARTICLE BioRisk www.pensoftonline.net/biorisk Myriapods (Myriapoda) Chapter 7.2 Pavel Stoev1, Marzio Zapparoli2, Sergei Golovatch3, Henrik Enghoff 4, Nesrine Akkari5, Anthony Barber6 1 National Museum of Natural History, Tsar Osvoboditel Blvd. 1, 1000 Sofi a, Bulgaria 2 Università degli Studi della Tuscia, Dipartimento di Protezione delle Piante, via S. Camillo de Lellis s.n.c., I-01100 Viterbo, Italy 3 Institute for Problems of Ecology and Evolution, Russian Academy of Sciences, Leninsky prospekt 33, Moscow 119071 Russia 4 Natural History Museum of Denmark (Zoological Museum), University of Copen- hagen, Universitetsparken 15, DK-2100 Copenhagen, Denmark 5 Research Unit of Biodiversity and Biology of Populations, Institut Supérieur des Sciences Biologiques Appliquées de Tunis, 9 Avenue Dr. Zouheir Essafi , La Rabta, 1007 Tunis, Tunisia 6 Rathgar, Exeter Road, Ivybridge, Devon, PL21 0BD, UK Corresponding author: Pavel Stoev ([email protected]) Academic editor: Alain Roques | Received 19 January 2010 | Accepted 21 May 2010 | Published 6 July 2010 Citation: Stoev P et al. -

Sexual Behaviour and Morphological Variation in the Millipede Megaphyllum Bosniense (Verhoeff, 1897)
Contributions to Zoology, 87 (3) 133-148 (2018) Sexual behaviour and morphological variation in the millipede Megaphyllum bosniense (Verhoeff, 1897) Vukica Vujić1, 2, Bojan Ilić1, Zvezdana Jovanović1, Sofija Pavković-Lučić1, Sara Selaković1, Vladimir Tomić1, Luka Lučić1 1 University of Belgrade, Faculty of Biology, Studentski Trg 16, 11000 Belgrade, Serbia 2 E-mail: [email protected] Keywords: copulation duration, Diplopoda, mating success, morphological traits, sexual behaviour, traditional and geometric morphometrics Abstract Analyses of morphological traits in M. bosniense ..........137 Discussion .............................................................................138 Sexual selection can be a major driving force that favours Morphological variation of antennae and legs morphological evolution at the intraspecific level. According between sexes with different mating status ......................143 to the sexual selection theory, morphological variation may Morphological variation of the head between sexes accompany non-random mating or fertilization. Here both with different mating status .............................................144 variation of linear measurements and variation in the shape Morphological variation of gonopods (promeres of certain structures can significantly influence mate choice in and opisthomeres) between males with different different organisms. In the present work, we quantified sexual mating status ....................................................................144 behaviour of the -

Fossil Calibrations for the Arthropod Tree of Life
bioRxiv preprint doi: https://doi.org/10.1101/044859; this version posted June 10, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. FOSSIL CALIBRATIONS FOR THE ARTHROPOD TREE OF LIFE AUTHORS Joanna M. Wolfe1*, Allison C. Daley2,3, David A. Legg3, Gregory D. Edgecombe4 1 Department of Earth, Atmospheric & Planetary Sciences, Massachusetts Institute of Technology, Cambridge, MA 02139, USA 2 Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, UK 3 Oxford University Museum of Natural History, Parks Road, Oxford OX1 3PZ, UK 4 Department of Earth Sciences, The Natural History Museum, Cromwell Road, London SW7 5BD, UK *Corresponding author: [email protected] ABSTRACT Fossil age data and molecular sequences are increasingly combined to establish a timescale for the Tree of Life. Arthropods, as the most species-rich and morphologically disparate animal phylum, have received substantial attention, particularly with regard to questions such as the timing of habitat shifts (e.g. terrestrialisation), genome evolution (e.g. gene family duplication and functional evolution), origins of novel characters and behaviours (e.g. wings and flight, venom, silk), biogeography, rate of diversification (e.g. Cambrian explosion, insect coevolution with angiosperms, evolution of crab body plans), and the evolution of arthropod microbiomes. We present herein a series of rigorously vetted calibration fossils for arthropod evolutionary history, taking into account recently published guidelines for best practice in fossil calibration.