The Coume Ouarnède System, a Hotspot of Subterranean Biodiversity in Pyrenees (France)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cylindroiulus Truncorum (Silvestri): a New Milliped for Virginia (USA), with Natural History Observations (Julida: Julidae)
Banisteria, Number 20, 2002 © 2002 by the Virginia Natural History Society Cylindroiulus truncorum (Silvestri): A New Milliped for Virginia (USA), with Natural History Observations (Julida: Julidae) Jorge A. Santiago-Blay Department of Paleobiology, MRC-121 National Museum of Natural History 10th and Constitution Avenue Smithsonian Institution P.O. Box 37012 Washington, DC 20013-7012 Richard L. Hoffman Virginia Museum of Natural History Martinsville, Virginia 24112 Joseph B. Lambert and Yuyang Wu Department of Chemistry Northwestern University 2145 Sheridan Road Evanston, Illinois 60208-3113 INTRODUCTION truncorum for Raleigh, North Carolina, about 320 km SSE of Salem (Shelley, 1978) is the southernmost In the fall 2000, author SB cleared the underbrush known occurrence of this species in the United States. of an Eastern White Pine (Pinus strobus L.) grove in his This milliped has also been documented for Brazil backyard located in an urban area of Salem, Virginia (Chamberlin & Hoffman, 1958; Hoffman, 1999). (USA) by cutting and removing the lower branches. About a year later, he revisited the same trees and Natural History Observations noticed copious resinous exudations originating from the branch stumps, particularly on five of the trees. Berlese extractions from P. strobus leaf litter were There, he observed about twenty millipeds, later conducted in November 2001 and yielded a maximum identified as Cylindroiulus truncorum (Silvestri, 1896; of about 50 C. truncorum per 0.25 m2 (= 200 C. species group reviewed by Korsós & Enghoff, 1990), truncorum per m2). In his many years of studying soil attached to the resin, 1-2 meters above ground (Fig. 1). invertebrates and running numerous Berlese samples, Voucher specimens of Cylindroiulus truncorum are particularly in southwestern Virginia, RLH has seldom deposited at the Virginia Museum of Natural History encountered millipeds under pine litter. -
Subterranean Biodiversity and Depth Distribution of Myriapods in Forested Scree Slopes of Central Europe
A peer-reviewed open-access journal ZooKeys Subterranean930: 117–137 (2020) biodiversity and depth distribution of myriapods in forested scree slopes of... 117 doi: 10.3897/zookeys.930.48914 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Subterranean biodiversity and depth distribution of myriapods in forested scree slopes of Central Europe Beáta Haľková1, Ivan Hadrián Tuf 2, Karel Tajovský3, Andrej Mock1 1 Institute of Biology and Ecology, Faculty of Science, Pavol Jozef Šafárik University, Košice, Slovakia 2 De- partment of Ecology and Environmental Sciences, Faculty of Science, Palacky University, Olomouc, Czech Republic 3 Institute of Soil Biology, Biology Centre CAS, České Budějovice, Czech Republic Corresponding author: Beáta Haľková ([email protected]) Academic editor: L. Dányi | Received 28 November 2019 | Accepted 10 February 2020 | Published 28 April 2020 http://zoobank.org/84BEFD1B-D8FA-4B05-8481-C0735ADF2A3C Citation: Haľková B, Tuf IH, Tajovský K, Mock A (2020) Subterranean biodiversity and depth distribution of myriapods in forested scree slopes of Central Europe. In: Korsós Z, Dányi L (Eds) Proceedings of the 18th International Congress of Myriapodology, Budapest, Hungary. ZooKeys 930: 117–137. https://doi.org/10.3897/zookeys.930.48914 The paper is dedicated to Christian Juberthie (12 Mar 1931–7 Nov 2019), the author of the concept of MSS (milieu souterrain superficiel) and the doyen of modern biospeleology Abstract The shallow underground of rock debris is a unique animal refuge. Nevertheless, the research of this habitat lags far behind the study of caves and soil, due to technical and time-consuming demands. Data on Myriapoda in scree habitat from eleven localities in seven different geomorphological units of the Czech and Slovak Republics were processed. -

Millipedes (Diplopoda) from Caves of Portugal
A.S.P.S. Reboleira and H. Enghoff – Millipedes (Diplopoda) from caves of Portugal. Journal of Cave and Karst Studies, v. 76, no. 1, p. 20–25. DOI: 10.4311/2013LSC0113 MILLIPEDES (DIPLOPODA) FROM CAVES OF PORTUGAL ANA SOFIA P.S. REBOLEIRA1 AND HENRIK ENGHOFF2 Abstract: Millipedes play an important role in the decomposition of organic matter in the subterranean environment. Despite the existence of several cave-adapted species of millipedes in adjacent geographic areas, their study has been largely ignored in Portugal. Over the last decade, intense fieldwork in caves of the mainland and the island of Madeira has provided new data about the distribution and diversity of millipedes. A review of millipedes from caves of Portugal is presented, listing fourteen species belonging to eight families, among which six species are considered troglobionts. The distribution of millipedes in caves of Portugal is discussed and compared with the troglobiont biodiversity in the overall Iberian Peninsula and the Macaronesian archipelagos. INTRODUCTION All specimens from mainland Portugal were collected by A.S.P.S. Reboleira, while collectors of Madeiran speci- Millipedes play an important role in the decomposition mens are identified in the text. Material is deposited in the of organic matter, and several species around the world following collections: Zoological Museum of University of have adapted to subterranean life, being found from cave Copenhagen, Department of Animal Biology, University of entrances to almost 2000 meters depth (Culver and Shear, La Laguna, Spain and in the collection of Sofia Reboleira, 2012; Golovatch and Kime, 2009; Sendra and Reboleira, Portugal. 2012). Although the millipede faunas of many European Species were classified according to their degree of countries are relatively well studied, this is not true of dependence on the subterranean environment, following Portugal. -

Comparative Functional Morphology of Attachment Devices in Arachnida
Comparative functional morphology of attachment devices in Arachnida Vergleichende Funktionsmorphologie der Haftstrukturen bei Spinnentieren (Arthropoda: Arachnida) DISSERTATION zur Erlangung des akademischen Grades doctor rerum naturalium (Dr. rer. nat.) an der Mathematisch-Naturwissenschaftlichen Fakultät der Christian-Albrechts-Universität zu Kiel vorgelegt von Jonas Otto Wolff geboren am 20. September 1986 in Bergen auf Rügen Kiel, den 2. Juni 2015 Erster Gutachter: Prof. Stanislav N. Gorb _ Zweiter Gutachter: Dr. Dirk Brandis _ Tag der mündlichen Prüfung: 17. Juli 2015 _ Zum Druck genehmigt: 17. Juli 2015 _ gez. Prof. Dr. Wolfgang J. Duschl, Dekan Acknowledgements I owe Prof. Stanislav Gorb a great debt of gratitude. He taught me all skills to get a researcher and gave me all freedom to follow my ideas. I am very thankful for the opportunity to work in an active, fruitful and friendly research environment, with an interdisciplinary team and excellent laboratory equipment. I like to express my gratitude to Esther Appel, Joachim Oesert and Dr. Jan Michels for their kind and enthusiastic support on microscopy techniques. I thank Dr. Thomas Kleinteich and Dr. Jana Willkommen for their guidance on the µCt. For the fruitful discussions and numerous information on physical questions I like to thank Dr. Lars Heepe. I thank Dr. Clemens Schaber for his collaboration and great ideas on how to measure the adhesive forces of the tiny glue droplets of harvestmen. I thank Angela Veenendaal and Bettina Sattler for their kind help on administration issues. Especially I thank my students Ingo Grawe, Fabienne Frost, Marina Wirth and André Karstedt for their commitment and input of ideas. -

Global Diversity of Marine Isopods (Except Asellota and Crustacean Symbionts)
Collection Review Global Diversity of Marine Isopods (Except Asellota and Crustacean Symbionts) Gary C. B. Poore1*, Niel L. Bruce2,3 1 Museum Victoria, Melbourne, Victoria, Australia, 2 Museum of Tropical Queensland and School of Marine and Tropical Biology, James Cook University, Townsville, Queensland, Australia, 3 Department of Zoology, University of Johannesburg, Auckland Park, South Africa known from the supralittoral and intertidal to depths in excess of Abstract: The crustacean order Isopoda (excluding six kilometres. Isopods are a highly diverse group of crustaceans, Asellota, crustacean symbionts and freshwater taxa) with more than 10,300 species known to date, approximately comprise 3154 described marine species in 379 genera 6,250 of these being marine or estuarine. In the groups under in 37 families according to the WoRMS catalogue. The discussion here (about half the species) the vast majority of species history of taxonomic discovery over the last two centuries are known from depths of less than 1000 metres. is reviewed. Although a well defined order with the Peracarida, their relationship to other orders is not yet The Isopoda is one of the orders of peracarid crustaceans, that resolved but systematics of the major subordinal taxa is is, those that brood their young in a marsupium under the body. relatively well understood. Isopods range in size from less They are uniquely defined within Peracarida by the combination than 1 mm to Bathynomus giganteus at 365 mm long. of one pair of uropods attached to the pleotelson and pereopods of They inhabit all marine habitats down to 7280 m depth only one branch. Marine isopods are arguably the most but with few doubtful exceptions species have restricted morphologically diverse order of all the Crustacea. -

Changes in Species Composition of Haploniscidae (Crustacea Isopoda)
Progress in Oceanography 180 (2020) 102233 Contents lists available at ScienceDirect Progress in Oceanography journal homepage: www.elsevier.com/locate/pocean Changes in species composition of Haploniscidae (Crustacea: Isopoda) across T potential barriers to dispersal in the Northwest Pacific ⁎ Nele Johannsena,b, Lidia Linsa,b,c, Torben Riehla,b, Angelika Brandta,b, a Goethe University, Biosciences, Institute for Ecology, Evolution und Diversity, Max-von-Laue-Str. 13, 60438 Frankfurt am Main, Germany b Senckenberg Research Institute and Natural History Museum, Marine Zoology, Senckenberganlage 25, 60325 Frankfurt am Main, Germany c Ghent University, Marine Biology Research Group, Krijgslaan 281/S8, 9000 Ghent, Belgium ARTICLE INFO ABSTRACT Keywords: Speciation processes as drivers of biodiversity in the deep sea are still not fully understood. One potential driver Hadal for species diversification might be allopatry caused by geographical barriers, such as ridges or trenches, or Ecology physiological barriers associated with depth. We analyzed biodiversity and biogeography of 21 morphospecies of Sea of Okhotsk the deep-sea isopod family Haploniscidae to investigate barrier effects to species dispersal in the Kuril- Deep sea Kamchatka Trench (KKT) area in the Northwest (NW) Pacific. Our study is based on 2652 specimens from three Abyssal genera, which were collected during the German-Russian KuramBio I (2012) and II (2016) expeditions as well as Isopoda Kuril Kamchatka Trench the Russian-German SokhoBio (2015) campaign. The sampling area covered two potential geographical barriers Distribution (the Kuril Island archipelago and the Kuril-Kamchatka Trench), as well as three depth zones (bathyal, abyssal, Northwest Pacific hadal). We found significant differences in relative species abundance between abyssal and hadal depths. -

Coleoptera) Deposited in the Natural History Museum of Barcelona, Spain
Arxius de Miscel·lània Zoològica, 12(2014): 13–82 ISSN:Viñolas 1698 & –Masó0476 The collection of type specimens of the family Carabidae (Coleoptera) deposited in the Natural History Museum of Barcelona, Spain A. Viñolas & G. Masó Viñolas, A. & Masó, G., 2014. The collection of type specimens of the family Carabidae (Coleoptera) deposited in the Natural History Museum of Barcelona, Spain. Arxius de Miscel·lània Zoològica, 12: 13–82. Abstract The collection of type specimens of the family Carabidae (Coleoptera) deposited in the Natural History Museum of Barcelona, Spain.— The type collection of the family Carabidae (Coleop- tera) deposited in the Natural History Museum of Barcelona, Spain, has been organised, revised and documented. It contains 430 type specimens belonging to 155 different taxa. Of note are the large number of hypogean species, the species of Cicindelidae from Asenci Codina’s collection, and the species of Harpalinae extracted from Jacques Nègre’s collec- tion. In this paper we provide all the available information related to these type specimens. We therefore provide the following information for each taxon, species or subspecies: the original and current taxonomic status, original citation of type materials, exact transcription of original labels, and preservation condition of specimens. Moreover, the differences between original descriptions and labels are discussed. When a taxonomic change has occurred, the references that examine those changes are included at the end of the taxa description. Key words: Collection type, Coleoptera, Carabidae taxonomic revision family, Ground beetles. Resumen La colección de ejemplares tipo de la familia Carabidae(Coleoptera) depositados en el Museo de Ciencias Naturales de Barcelona, España.— Se ha organizado, revisado y documentado la colección de especímenes tipo de la familia Carabidae (Coleoptera) de- positados en el Museo de Ciencias Naturales de Barcelona. -

From Ghost and Mud Shrimp
Zootaxa 4365 (3): 251–301 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2017 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4365.3.1 http://zoobank.org/urn:lsid:zoobank.org:pub:C5AC71E8-2F60-448E-B50D-22B61AC11E6A Parasites (Isopoda: Epicaridea and Nematoda) from ghost and mud shrimp (Decapoda: Axiidea and Gebiidea) with descriptions of a new genus and a new species of bopyrid isopod and clarification of Pseudione Kossmann, 1881 CHRISTOPHER B. BOYKO1,4, JASON D. WILLIAMS2 & JEFFREY D. SHIELDS3 1Division of Invertebrate Zoology, American Museum of Natural History, Central Park West @ 79th St., New York, New York 10024, U.S.A. E-mail: [email protected] 2Department of Biology, Hofstra University, Hempstead, New York 11549, U.S.A. E-mail: [email protected] 3Department of Aquatic Health Sciences, Virginia Institute of Marine Science, College of William & Mary, P.O. Box 1346, Gloucester Point, Virginia 23062, U.S.A. E-mail: [email protected] 4Corresponding author Table of contents Abstract . 252 Introduction . 252 Methods and materials . 253 Taxonomy . 253 Isopoda Latreille, 1817 . 253 Bopyroidea Rafinesque, 1815 . 253 Ionidae H. Milne Edwards, 1840. 253 Ione Latreille, 1818 . 253 Ione cornuta Bate, 1864 . 254 Ione thompsoni Richardson, 1904. 255 Ione thoracica (Montagu, 1808) . 256 Bopyridae Rafinesque, 1815 . 260 Pseudioninae Codreanu, 1967 . 260 Acrobelione Bourdon, 1981. 260 Acrobelione halimedae n. sp. 260 Key to females of species of Acrobelione Bourdon, 1981 . 262 Gyge Cornalia & Panceri, 1861. 262 Gyge branchialis Cornalia & Panceri, 1861 . 262 Gyge ovalis (Shiino, 1939) . 264 Ionella Bonnier, 1900 . -
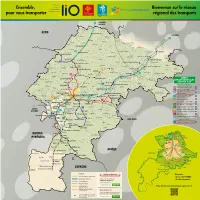
Lignes Régulières
Ensemble, Bienvenue sur le réseau pour vous transporter régional des transports SAMATAN TOULOUSE Boissède Molas GERS TOULOUSE Puymaurin Ambax Sénarens Riolas Nénigan D 17 Pouy-de-Touges Lunax Saint-Frajou Gratens Péguilhan Fabas 365 Castelnau-Picampeau Polastron Latte-Vigordane Lussan-Adeilhac Le Fousseret Lacaugne Eoux Castéra-Vignoles Francon Rieux-Volvestre Boussan Latrape 342 Mondavezan Nizan-Gesse Lespugue 391 D 62 Bax Castagnac Sarrecave Marignac-Laspeyres Goutevernisse Canens Sarremezan 380 Montesquieu-Volvestre 344 Peyrouzet D 8 Larroque Saint- LIGNES Christaud Gouzens Cazaril-Tamboures DÉPARTEMENTALES Montclar-de-Comminges Saint-Plancard Larcan SECTEUR SUD Auzas Le Plan D 5 320 Lécussan Lignes régulières Sédeilhac Sepx Montberaud Ausseing 342 L’ISLE-EN-DODON - SAINT-GAUDENS Le Cuing Lahitère 344 BOULOGNE - SAINT-GAUDENS Franquevielle Landorthe 365 BOULOGNE - L'ISLE-EN-DODON - Montbrun-Bocage SAMATAN - TOULOUSE Les Tourreilles 379 LAVELANET - MAURAN - CAZÈRES 394 D 817 379 SAINT-GAUDENS 397 380 CAZÈRES - NOÉ - TOULOUSE D 117 391 ALAN - AURIGNAC - SAINT-GAUDENS Touille 392 MONCAUP - ASPET - SAINT-GAUDENS His 393 392 Ganties MELLES - SAINT-BÉAT - SAINT-GAUDENS TARBES D 9 Rouède 394 LUCHON - MONTRÉJEAU - SAINT-GAUDENS N 125 398 BAYONNE 395 LES(Val d’Aran) - BARBAZAN - SAINT-GAUDENS Castelbiague Estadens 397 MANE - SAINT-GAUDENS Saleich 398 MONTRÉJEAU - BARBAZAN - SAINT-GAUDENS Chein-Dessus SEPTEMBRE 2019 - CD31/19/7/42845 395 393 Urau Francazal SAINT-GIRONS Navette SNCF D 5 Arbas 320 AURIGNAC - SAINT-MARTORY - BOUSSENS SNCF Fougaron -

Tectonic Vicariance Versus Messinian Dispersal in Western Mediterranean
1 Preprint of a manuscript accepted for publication in Zoologica Scripta 2 3 Tectonic vicariance versus Messinian dispersal in western 4 Mediterranean ground beetles (Carabidae Trechini and 5 Pterostichini Molopina) 6 7 1,2 3 4 5 1 8 Arnaud Faille , Achille Casale , Carles Hernando , Salah Aït Mouloud and Ignacio Ribera 9 1 10 Institute of Evolutionary Biology (CSIC-Universitat Pompeu Fabra), Passeig Maritim de la 11 Barceloneta 37, 08003 Barcelona, Spain 2 12 MECADEV - UMR 7179 MNHN/CNRS, Paris, France 3 13 C/o Università di Sassari, Dipartimento di Scienze della Natura e del Territorio (Zoologia). 14 Private: Corso Raffaello 12, 10126 Torino, Italy. e-mail: [email protected] 4 15 P.O. box 118, 08911 Badalona, Catalonia, Spain 5 16 Université Mouloud-Mammeri, Tizi Ouzou, Algeria 17 18 19 Correspondence: A. Faille, Institut de Biologia Evolutiva (CSIC-Universitat Pompeu Fabra), 20 Barcelona, Spain. E-mail: [email protected] 21 1 22 ABSTRACT 23 24 The complex geological history of the western Mediterranean region complicates the 25 interpretation of the evolutionary history of its current fauna, as similar distribution patterns 26 may have very different temporal and geographical origins. Particularly intriguing are some 27 subterranean species in islands, which origin is usually difficult to interpret as their strongly 28 modified morphologies obscure their relationships. We studied subterranean taxa and their 29 likely relatives of two groups of ground beetles in the western Mediterranean: the Duvalius 30 lineage ("isotopic" Trechini) and Molopina (Pterostichini). We included specimens from the 31 islands of Mallorca, Sardinia and Sicily, plus mainland Europe and north Africa. -

Allopatric Speciation Illustrated
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Arthropod Systematics and Phylogeny Jahr/Year: 2015 Band/Volume: 73 Autor(en)/Author(s): Faille Arnaud, Bourdeau Charles, Belles Xavier, Fresneda Javier Artikel/Article: Allopatric speciation illustrated: The hypogean genus Geotrechus Jeannel, 1919 (Coleoptera: Carabidae: Trechini), with description of four new species from the Eastern Pyrenees (Spain) 439-455 73 (3): 439 – 455 23.12.2015 © Senckenberg Gesellschaft für Naturforschung, 2015. Allopatric speciation illustrated: The hypogean genus Geotrechus Jeannel, 1919 (Coleoptera: Carabidae: Trechini), with description of four new species from the Eastern Pyrenees (Spain) Arnaud Faille *, 1, Charles Bourdeau 2, Xavier Belles 3 & Javier Fresneda 4 1 Zoologische Staatssammlung München, Münchhausenstraße 21, 81247 Munich, Germany; Arnaud Faille [[email protected]] — 2 5 che min FournierHaut, F31320 Rebigue, France; Charles Bourdeau [[email protected]] — 3 Institute of Evolutionary Biology (CSICUniver sitat Pompeu Fabra), Passeig Maritim de la Barceloneta 37, E08003 Barcelona, Spain; Xavier Belles [[email protected]csic.es] — 4 Ca de Massa, 25526 Llesp – El Pont de Suert, Lleida, Spain / Museu de Ciències Naturals (Zoologia), Passeig Picasso s/n, 08003 Barcelona, Spain; Javier Fresneda [ffresned @gmail.com] — * Correspond ing author Accepted 09.x.2015. Published online at www.senckenberg.de/arthropodsystematics on 14.xii.2015. Editor in charge: Steffen Pauls. Abstract We present a study of the eastern group of species of the genus Geotrechus (Coleoptera, Carabidae, Trechini), combining molecular and morphological approaches. Four new species are described from caves of the Pyrenees of Catalonia, Spain. Two of the new species belong to the Geotrechus ubachi group sensu novo, like all the species previously known. -

Haute-Garonne - Dépistage Tuberculose - Camapgne 2018-2019
Liste communes département Haute-Garonne - Dépistage Tuberculose - Camapgne 2018-2019 Source: DDPP31 MAJ : 24/10/2018 CP Commune Canton Info Zone spécifique 31037 AVIGNONET-LAURAGAIS VILLEFRANCHE 31038 AZAS MONTASTRUC 31054 BEAUTEVILLE VILLEFRANCHE 31066 BESSIERES MONTASTRUC 31074 BONREPOS-RIQUET VERFEIL 31078 BOUDRAC MONTREJEAU ZR 31094 BUZET-SUR-TARN MONTASTRUC 31103 CANENS MONTESQUIEU ZR 31111 CASTAGNAC MONTESQUIEU ZR 31119 CASTELNAU-PICAMPEAU LE FOUSSERET 31122 CASTIES-LABRANDE LE FOUSSERET 31137 CESSALES VILLEFRANCHE 31145 CINTEGABELLE CINTEGABELLE 31173 ESPERCE CINTEGABELLE 31184 FLOURENS TOULOUSE 31193 LE FOUSSERET LE FOUSSERET 31226 GOUZENS MONTESQUIEU ZR 31267 LAHITERE MONTESQUIEU ZR 31272 LAPEYRERE MONTESQUIEU ZR 31279 LATOUR MONTESQUIEU ZR 31320 MARQUEFAVE CARBONNE ZR 31326 MASSABRAC CARBONNE ZR 31365 MONTBRUN-BOCAGE MONTESQUIEU ZR 31375 MONTESQUIEU-VOLVESTRE MONTESQUIEU ZR 31379 MONTGAZIN CARBONNE ZR 31428 POLASTRON LE FOUSSERET 31436 POUY-DE-TOUGES LE FOUSSERET 31469 SAINT-ARAILLE LE FOUSSERET 31474 SAINT-CHRISTAUD MONTESQUIEU 31476 SAINT-ELIX-LE-CHATEAU LE FOUSSERET 31486 SAINT-HILAIRE MURET 31543 SENARENS LE FOUSSERET 31547 SEYSSES MURET 31555 TOULOUSE TOULOUSE 31589 VILLENOUVELLE VILLEFRANCHE 31001 AGASSAC ISLE EN DODON 31005 ALAN AURIGNAC 31006 ALBIAC CARAMAN 31007 AMBAX ISLE EN DODON 31008 ANAN ISLE EN DODON 31009 ANTICHAN-DE-FRONTIGNES BARBAZAN 31010 ANTIGNAC LUCHON 31011 ARBAS ASPET 31012 ARBON ASPET 31013 ARDIEGE BARBAZAN 31014 ARGUENOS ASPET 31015 ARGUT-DESSOUS SAINT-BEAT 31017 ARLOS SAINT-BEAT 31019 ARTIGUE LUCHON