COPYRIGHTED MATERIAL Extremophiles Have Evolved to Adapt to Severe Geological Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Taxonomic and Chemical Assessment of Exceptionally Abundant Rock Mine Biofilm
Taxonomic and chemical assessment of exceptionally abundant rock mine biofilm Karolina Tomczyk-Żak1, Paweª Szczesny2,3, Robert Gromadka4 and Urszula Zielenkiewicz1 1 Department of Microbial Biochemistry, Institute of Biochemistry and Biophysics, PAS, Warsaw, Poland 2 Faculty of Biology, Institute of Experimental Plant Biology and Biotechnology, University of Warsaw, Warsaw, Poland 3 Department of Bioinformatics, Institute of Biochemistry and Biophysics Polish Academy of Sciences, Warsaw, Poland 4 Laboratory of DNA Sequencing and Oligonucleotides Synthesis, Institute of Biochemistry and Biophysics, PAS, Warsaw, Poland ABSTRACT Background. An exceptionally thick biofilm covers walls of ancient gold and arsenic Zªoty Stok mine (Poland) in the apparent absence of organic sources of energy. Methods and Results. We have characterized this microbial community using culture- dependent and independent methods. We sequenced amplicons of the 16S rRNA gene obtained using generic primers and additional primers targeted at Archaea and Actinobacteria separately. Also, we have cultured numerous isolates from the biofilm on different media under aerobic and anaerobic conditions. We discovered very high biodiversity, and no single taxonomic group was dominant. The majority of almost 4,000 OTUs were classified above genus level indicating presence of novel species. Elemental analysis, performed using SEM-EDS and X-ray, of biofilm samples showed that carbon, sulphur and oxygen were not evenly distributed in the biofilm and that their presence is highly correlated. However, the distribution of arsenic and iron was more flat, and numerous intrusions of elemental silver and platinum were noted, indicating that microorganisms play a key role in releasing these elements from the rock. Submitted 13 April 2017 Conclusions. -

Venter Institute
Genomics:GTL Program Projects J. Craig Venter Institute 42 Estimation of the Minimal Mycoplasma Gene Set Using Global Transposon Mutagenesis and Comparative Genomics John I. Glass* ([email protected]), Nina Alperovich, Nacyra Assad-Garcia, Shibu Yooseph, Mahir Maruf, Carole Lartigue, Cynthia Pfannkoch, Clyde A. Hutchison III, Hamilton O. Smith, and J. Craig Venter J. Craig Venter Institute, Rockville, MD The Venter Institute aspires to make bacteria with specific metabolic capabilities encoded by artificial genomes. To achieve this we must develop technologies and strategies for creating bacterial cells from constituent parts of either biological or synthetic origin. Determining the minimal gene set needed for a functioning bacterial genome in a defined laboratory environment is a necessary step towards our goal. For our initial rationally designed cell we plan to synthesize a genome based on a mycoplasma blueprint (mycoplasma being the common name for the class Mollicutes). We chose this bacterial taxon because its members already have small, near minimal genomes that encode limited metabolic capacity and complexity. We took two approaches to determine what genes would need to be included in a truly minimal synthetic chromosome of a planned Mycoplasma laboratorium: determination of all the non-essential genes through random transposon mutagenesis of model mycoplasma species, Mycoplasma genitalium, and comparative genomics of a set of 15 mycoplasma genomes in order to identify genes common to all members of the taxon. Global transposon mutagenesis has been used to predict the essential gene set for a number of bac- teria. In Bacillus subtilis all but 271 of bacterium’s ~4100 genes could be knocked out. -

Methanothermus Fervidus Type Strain (V24S)
UC Davis UC Davis Previously Published Works Title Complete genome sequence of Methanothermus fervidus type strain (V24S). Permalink https://escholarship.org/uc/item/9367m39j Journal Standards in genomic sciences, 3(3) ISSN 1944-3277 Authors Anderson, Iain Djao, Olivier Duplex Ngatchou Misra, Monica et al. Publication Date 2010-11-20 DOI 10.4056/sigs.1283367 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Standards in Genomic Sciences (2010) 3:315-324 DOI:10.4056/sigs.1283367 Complete genome sequence of Methanothermus fervidus type strain (V24ST) Iain Anderson1, Olivier Duplex Ngatchou Djao2, Monica Misra1,3, Olga Chertkov1,3, Matt Nolan1, Susan Lucas1, Alla Lapidus1, Tijana Glavina Del Rio1, Hope Tice1, Jan-Fang Cheng1, Roxanne Tapia1,3, Cliff Han1,3, Lynne Goodwin1,3, Sam Pitluck1, Konstantinos Liolios1, Natalia Ivanova1, Konstantinos Mavromatis1, Natalia Mikhailova1, Amrita Pati1, Evelyne Brambilla4, Amy Chen5, Krishna Palaniappan5, Miriam Land1,6, Loren Hauser1,6, Yun-Juan Chang1,6, Cynthia D. Jeffries1,6, Johannes Sikorski4, Stefan Spring4, Manfred Rohde2, Konrad Eichinger7, Harald Huber7, Reinhard Wirth7, Markus Göker4, John C. Detter1, Tanja Woyke1, James Bristow1, Jonathan A. Eisen1,8, Victor Markowitz5, Philip Hugenholtz1, Hans-Peter Klenk4, and Nikos C. Kyrpides1* 1 DOE Joint Genome Institute, Walnut Creek, California, USA 2 HZI – Helmholtz Centre for Infection Research, Braunschweig, Germany 3 Los Alamos National Laboratory, Bioscience Division, Los Alamos, New Mexico, USA 4 DSMZ - German Collection of Microorganisms and Cell Cultures GmbH, Braunschweig, Germany 5 Biological Data Management and Technology Center, Lawrence Berkeley National Laboratory, Berkeley, California, USA 6 Oak Ridge National Laboratory, Oak Ridge, Tennessee, USA 7 University of Regensburg, Archaeenzentrum, Regensburg, Germany 8 University of California Davis Genome Center, Davis, California, USA *Corresponding author: Nikos C. -

Microbial Identification Framework for Risk Assessment
Microbial Identification Framework for Risk Assessment May 2017 Cat. No.: En14-317/2018E-PDF ISBN 978-0-660-24940-7 Information contained in this publication or product may be reproduced, in part or in whole, and by any means, for personal or public non-commercial purposes, without charge or further permission, unless otherwise specified. You are asked to: • Exercise due diligence in ensuring the accuracy of the materials reproduced; • Indicate both the complete title of the materials reproduced, as well as the author organization; and • Indicate that the reproduction is a copy of an official work that is published by the Government of Canada and that the reproduction has not been produced in affiliation with or with the endorsement of the Government of Canada. Commercial reproduction and distribution is prohibited except with written permission from the author. For more information, please contact Environment and Climate Change Canada’s Inquiry Centre at 1-800-668-6767 (in Canada only) or 819-997-2800 or email to [email protected]. © Her Majesty the Queen in Right of Canada, represented by the Minister of the Environment and Climate Change, 2016. Aussi disponible en français Microbial Identification Framework for Risk Assessment Page 2 of 98 Summary The New Substances Notification Regulations (Organisms) (the regulations) of the Canadian Environmental Protection Act, 1999 (CEPA) are organized according to organism type (micro- organisms and organisms other than micro-organisms) and by activity. The Microbial Identification Framework for Risk Assessment (MIFRA) provides guidance on the required information for identifying micro-organisms. This document is intended for those who deal with the technical aspects of information elements or information requirements of the regulations that pertain to identification of a notified micro-organism. -

A Proposal to Rename the Hyperthermophile Pyrococcus Woesei As Pyrococcus Furiosus Subsp
Archaea 1, 277–283 © 2004 Heron Publishing—Victoria, Canada A proposal to rename the hyperthermophile Pyrococcus woesei as Pyrococcus furiosus subsp. woesei WIROJNE KANOKSILAPATHAM,1 JUAN M. GONZÁLEZ,1,2 DENNIS L. MAEDER,1 1,3 1,4 JOCELYNE DIRUGGIERO and FRANK T. ROBB 1 Center of Marine Biotechnology, University of Maryland Biotechnology Institute, Baltimore, MD 21202, USA 2 Present address: IRNAS-CSIC, P.O. Box 1052, 41080 Sevilla, Spain 3 Department of Cell Biology and Molecular Genetics, University of Maryland, College Park, MD 20274, USA 4 Corresponding author ([email protected]) Received April 8, 2004; accepted July 28, 2004; published online August 31, 2004 Summary Pyrococcus species are hyperthermophilic mem- relatively rapidly at temperatures above 90 °C and produce ° bers of the order Thermococcales, with optimal growth tem- H2S from elemental sulfur (S ) (Zillig et al. 1987). Two Pyro- peratures approaching 100 °C. All species grow heterotrophic- coccus species, P. furiosus (Fiala and Stetter 1986) and ° ally and produce H2 or, in the presence of elemental sulfur (S ), P. woesei (Zillig et al. 1987), were isolated from marine sedi- H2S. Pyrococcus woesei and P.furiosus were isolated from ma- ments at a Vulcano Island beach site in Italy. They have similar rine sediments at the same Vulcano Island beach site and share morphological and physiological characteristics: both species many morphological and physiological characteristics. We re- are cocci and move by means of a tuft of polar flagella. They port here that the rDNA operons of these strains have identical are heterotrophic and grow optimally at 95–100 °C, utilizing sequences, including their intergenic spacer regions and part of peptides as major carbon and nitrogen sources. -

Role of Protein Phosphorylation in Mycoplasma Pneumoniae
Pathogenicity of a minimal organism: Role of protein phosphorylation in Mycoplasma pneumoniae Dissertation zur Erlangung des mathematisch-naturwissenschaftlichen Doktorgrades „Doctor rerum naturalium“ der Georg-August-Universität Göttingen vorgelegt von Sebastian Schmidl aus Bad Hersfeld Göttingen 2010 Mitglieder des Betreuungsausschusses: Referent: Prof. Dr. Jörg Stülke Koreferent: PD Dr. Michael Hoppert Tag der mündlichen Prüfung: 02.11.2010 “Everything should be made as simple as possible, but not simpler.” (Albert Einstein) Danksagung Zunächst möchte ich mich bei Prof. Dr. Jörg Stülke für die Ermöglichung dieser Doktorarbeit bedanken. Nicht zuletzt durch seine freundliche und engagierte Betreuung hat mir die Zeit viel Freude bereitet. Des Weiteren hat er mir alle Freiheiten zur Verwirklichung meiner eigenen Ideen gelassen, was ich sehr zu schätzen weiß. Für die Übernahme des Korreferates danke ich PD Dr. Michael Hoppert sowie Prof. Dr. Heinz Neumann, PD Dr. Boris Görke, PD Dr. Rolf Daniel und Prof. Dr. Botho Bowien für das Mitwirken im Thesis-Komitee. Der Studienstiftung des deutschen Volkes gilt ein besonderer Dank für die finanzielle Unterstützung dieser Arbeit, durch die es mir unter anderem auch möglich war, an Tagungen in fernen Ländern teilzunehmen. Prof. Dr. Michael Hecker und der Gruppe von Dr. Dörte Becher (Universität Greifswald) danke ich für die freundliche Zusammenarbeit bei der Durchführung von zahlreichen Proteomics-Experimenten. Ein ganz besonderer Dank geht dabei an Katrin Gronau, die mich in die Feinheiten der 2D-Gelelektrophorese eingeführt hat. Außerdem möchte ich mich bei Andreas Otto für die zahlreichen Proteinidentifikationen in den letzten Monaten bedanken. Nicht zu vergessen ist auch meine zweite Außenstelle an der Universität in Barcelona. Dr. Maria Lluch-Senar und Dr. -

And Thermo-Adaptation in Hyperthermophilic Archaea: Identification of Compatible Solutes, Accumulation Profiles, and Biosynthetic Routes in Archaeoglobus Spp
Universidade Nova de Lisboa Osmo- andInstituto thermo de Tecnologia-adaptation Química e Biológica in hyperthermophilic Archaea: Subtitle Subtitle Luís Pedro Gafeira Gonçalves Osmo- and thermo-adaptation in hyperthermophilic Archaea: identification of compatible solutes, accumulation profiles, and biosynthetic routes in Archaeoglobus spp. OH OH OH CDP c c c - CMP O O - PPi O3P P CTP O O O OH OH OH OH OH OH O- C C C O P O O P i Dissertation presented to obtain the Ph.D degree in BiochemistryO O- Instituto de Tecnologia Química e Biológica | Universidade Nova de LisboaP OH O O OH OH OH Oeiras, Luís Pedro Gafeira Gonçalves January, 2008 2008 Universidade Nova de Lisboa Instituto de Tecnologia Química e Biológica Osmo- and thermo-adaptation in hyperthermophilic Archaea: identification of compatible solutes, accumulation profiles, and biosynthetic routes in Archaeoglobus spp. This dissertation was presented to obtain a Ph. D. degree in Biochemistry at the Instituto de Tecnologia Química e Biológica, Universidade Nova de Lisboa. By Luís Pedro Gafeira Gonçalves Supervised by Prof. Dr. Helena Santos Oeiras, January, 2008 Apoio financeiro da Fundação para a Ciência e Tecnologia (POCI 2010 – Formação Avançada para a Ciência – Medida IV.3) e FSE no âmbito do Quadro Comunitário de apoio, Bolsa de Doutoramento com a referência SFRH / BD / 5076 / 2001. ii ACKNOWNLEDGMENTS The work presented in this thesis, would not have been possible without the help, in terms of time and knowledge, of many people, to whom I am extremely grateful. Firstly and mostly, I need to thank my supervisor, Prof. Helena Santos, for her way of thinking science, her knowledge, her rigorous criticism, and her commitment to science. -

Ana Maria Da Silva Esteves Dissertation Presented to Obtain The
Insights into the biochemical strategies of adaptation to heat stress in the hyperthermophilic archaeon Pyrococcus furiosus Ana Maria da Silva Esteves Dissertation presented to obtain the Ph.D. degree in Biochemistry Instituto de Tecnologia Química e Biológica António Xavier | Universidade Nova de Lisboa Oeiras, February, 2015 Insights into the biochemical strategies of adaptation to heat stress in the hyperthermophilic archaeon Pyrococcus furiosus Ana Maria da Silva Esteves Supervisor: Professora Helena Santos Co-supervisor: Dr. Nuno Borges Dissertation presented to obtain the Ph.D degree in Biochemistry Instituto de Tecnologia Química e Biológica António Xavier | Universidade Nova de Lisboa Oeiras, February, 2015 From left to right: Prof. Volker Müller, Dr. Emmanouil Matzapetakis, Dr. Nuno Borges (Co-supervisor), Ana M. Esteves, Prof. Helena Santos (Supervisor), Prof. Pedro Moradas Ferreira, Prof. Beate Averhoff, Prof. Hermínia de Lencastre. Oeiras, 9th of February 2015. Apoio financeiro da Fundação para a Ciência e Tecnologia e do FSE no âmbito do Quadro Comunitário de apoio, Bolsa de Doutoramento com a referência SFRH/BD/61742/2009. In loving memory of my grandmother Elisa iv Acknowledgements First, I would like to thank Prof. Helena Santos, my supervisor, for accepting me as a Ph.D. student in her laboratory. Also, I want to express my appreciation to Prof. Helena Santos for being an excellent team leader, and for her constant effort to put together the resources and expertise her students need to carry out their projects with success. Her guidance, rigor, patience and enthusiasm for science definitely contributed to my development as a young scientist. I want to thank Dr. -
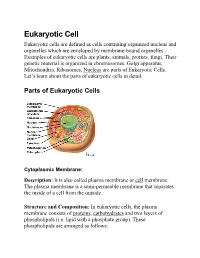
Eukaryotic Cell Eukaryotic Cells Are Defined As Cells Containing Organized Nucleus and Organelles Which Are Enveloped by Membrane-Bound Organelles
Eukaryotic Cell Eukaryotic cells are defined as cells containing organized nucleus and organelles which are enveloped by membrane-bound organelles. Examples of eukaryotic cells are plants, animals, protists, fungi. Their genetic material is organized in chromosomes. Golgi apparatus, Mitochondria, Ribosomes, Nucleus are parts of Eukaryotic Cells. Let’s learn about the parts of eukaryotic cells in detail. Parts ot Eukaryotic Cells Cytoplasmic Membrane: Description: It is also called plasma membrane or cell membrane. The plasma membrane is a semi-permeable membrane that separates the inside of a cell from the outside. Structure and Composition: In eukaryotic cells, the plasma membrane consists of proteins , carbohydrates and two layers of phospholipids (i.e. lipid with a phosphate group). These phospholipids are arranged as follows: • The polar, hydrophilic (water-loving) heads face the outside and inside of the cell. These heads interact with the aqueous environment outside and within a cell. • The non-polar, hydrophobic (water-repelling) tails are sandwiched between the heads and are protected from the aqueous environments. Scientists Singer and Nicolson(1972) described the structure of the phospholipid bilayer as the ‘Fluid Mosaic Model’. The reason is that the bi-layer looks like a mosaic and has a semi-fluid nature that allows lateral movement of proteins within the bilayer. Image: Fluid mosaic model. Orange circles – Hydrophilic heads; Lines below – Hydrophobic tails. Functions • The plasma membrane is selectively permeable i.e. it allows only selected substances to pass through. • It protects the cells from shock and injuries. • The fluid nature of the membrane allows the interaction of molecules within the membrane. -

Microbial Community Composition in Acid Mine Drainage Lake of Xiang
This article was downloaded by: [Zhejiang University] On: 11 April 2013, At: 17:31 Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Geomicrobiology Journal Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/ugmb20 Microbial Community Composition in Acid Mine Drainage Lake of Xiang Mountain Sulfide Mine in Anhui Province, China Chunbo Hao a b , Lina Zhang a b , Lihua Wang a b , Siyuan Li a b & Hailiang Dong a c a Geomicrobiology Laboratory, State Key Laboratory of Geobiology and Environmental Geology, China University of Geosciences, Beijing, China b School of Water Resources and Environment, China University of Geosciences, Beijing, China c Department of Geology and Environmental Earth Science, Miami University, Oxford, Ohio, USA Accepted author version posted online: 18 Apr 2012.Version of record first published: 14 Sep 2012. To cite this article: Chunbo Hao , Lina Zhang , Lihua Wang , Siyuan Li & Hailiang Dong (2012): Microbial Community Composition in Acid Mine Drainage Lake of Xiang Mountain Sulfide Mine in Anhui Province, China, Geomicrobiology Journal, 29:10, 886-895 To link to this article: http://dx.doi.org/10.1080/01490451.2011.635762 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.tandfonline.com/page/terms-and-conditions This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. -

Chemolithotrophic Nitrate-Dependent Fe(II)-Oxidizing Nature of Actinobacterial Subdivision Lineage TM3
The ISME Journal (2013) 7, 1582–1594 & 2013 International Society for Microbial Ecology All rights reserved 1751-7362/13 www.nature.com/ismej ORIGINAL ARTICLE Chemolithotrophic nitrate-dependent Fe(II)-oxidizing nature of actinobacterial subdivision lineage TM3 Dheeraj Kanaparthi1, Bianca Pommerenke1, Peter Casper2 and Marc G Dumont1 1Department of Biogeochemistry, Max Planck Institute for Terrestrial Microbiology, Marburg, Germany and 2Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Department of Limnology of Stratified Lakes, Stechlin, Germany Anaerobic nitrate-dependent Fe(II) oxidation is widespread in various environments and is known to be performed by both heterotrophic and autotrophic microorganisms. Although Fe(II) oxidation is predominantly biological under acidic conditions, to date most of the studies on nitrate-dependent Fe(II) oxidation were from environments of circumneutral pH. The present study was conducted in Lake Grosse Fuchskuhle, a moderately acidic ecosystem receiving humic acids from an adjacent bog, with the objective of identifying, characterizing and enumerating the microorganisms responsible for this process. The incubations of sediment under chemolithotrophic nitrate- dependent Fe(II)-oxidizing conditions have shown the enrichment of TM3 group of uncultured Actinobacteria. A time-course experiment done on these Actinobacteria showed a consumption of Fe(II) and nitrate in accordance with the expected stoichiometry (1:0.2) required for nitrate- dependent Fe(II) oxidation. Quantifications done by most probable number showed the presence of 1 Â 104 autotrophic and 1 Â 107 heterotrophic nitrate-dependent Fe(II) oxidizers per gram fresh weight of sediment. The analysis of microbial community by 16S rRNA gene amplicon pyrosequencing showed that these actinobacterial sequences correspond to B0.6% of bacterial 13 16S rRNA gene sequences. -

Appendix 1*) for Essential Information Very Well Illustrated in Google and Wikipedia
Appendix 1*) For Essential Information Very Well Illustrated in Google and Wikipedia *) in Support of the Text with Literature Citations. Referrals to illustrations in Appendix 2. Cancer in the Plant. The insertion of the Agrobacterium tumefaciens circular plasmid T (transferred) DNA into the genome of its new host, the plant (Gelvin BS. Microbiol Molecular Biol Rev 2003;67:16–37). The plant cancer “crown gall” (agrocallus; Agrobacterial crown gall) consists of malignantly transformed cells replicating the agrobacterial T DNA plasmid (reviewed in postscript Table XXXV). For reference: Koncz C Mayerhofer R Koncz-Kálmán Zs et al EMBO J 1990;9:1337–1346. Transfer of potentially oncogenic bacterial genes and proteins to patients: Septicemic Bacteroides enterotoxigenic (Sinkovics J G & Smith JP Cancer 1970;25:663–671; Viljoen KS et al PLoS One 2015;10(3):e0119462); Bartonella bacilliformis etc (Guy L et al PLoS Genet 2013;9(3):e1003393; Harms A & Dehio C Clin Microbiol Rev 2012;25:42–78; Llosa M et al Trends Microbiol 2012;20:355–9; Minnick MF et al PLoS Negl Trop Dis 2014;6(7):e2919); Helicobacter pylori (Bonsor DA et al J Biol Chem 2015;pii:jbc.M115.641829; Su YL et al J Immunol 2015;194:3997–4007; Vaziri F et al Pathog Dis 2015;73(3). pii.ftu021); Porphyromonas gingivalis (Katz J et al Int J Oral Sci 2011;3:209–215); Tuberculous infections with A. tumefaciens in patients (Ramirez FC et al Clin Infect Dis 1992;15:938–940). DNA-binding Antibodies. DNA- (or RNA-) binding proteins use zink finger motifs, leucine zippers and winged (beta-sheet loops) helix-turn helix motifs (HTH, two helices separated by the loop, RNA/DNA-binding domains) in recognition of RNA/DNA receptors for attachment.