Labeling of Processed and Blended Seafood Products Made Primarily with Fish Protein
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appetizer Kuzu Tofu, Crab Meat, Field Caviar and Chrysanthemum Petals
RAN Appetizer Kuzu Tofu, Crab Meat, Field Caviar and Chrysanthemum Petals with Dashi Jelly Salmon Seasoned with Kelp, Cucumber, Salmon Roe Boiled and Seasoned Chinese Cabbage, Chrysanthemum Leaves and Mushroom Soup Soy Milk Skin Dumpling, Whale, Naruto Wakame Brown Seaweed Sashimi A Selection of Seasonal Sashimi (Change 3 kinds of sashimi to 5 kinds, additional charge of 1,500yen) Broiled Dish Broiled Splendid Alfonsino Seasoned with Shichimi Peppers Vinegared Vegetables Organic Beef Fillet with Vegetables Japanese Sauce and Whole-grain Mustard Fried Dish Assorted Tempura Braised Dish Simmered Turnip, Fried and Simmered Taro, Maitake Mushroom, Carrot Green with Yoshino Miso Rice Dish Nigiri Sushi and Rolled Sushi with Miso Soup (To be upgraded with additional charge of 1,500yen) Dessert Assorted Seasonal Dessert with Wine Jelly ¥11,000 The menu may change without prior notice. We use domestic rice. Please notify us in advance if you have any allergy to specific food items such as gluten or lactose. 13% service charge and consumption tax will be added to your bill. MIYABI Appetizer Grilled Anago Conger, Cucumber, Maitake Mushroom Radish with Ponzu Jelly Steamed Dish Mini Egg Custard: Crab Meat, Soy Milk Skin, Lily Bulb and Starchy Dashi Sauce Sashimi A Selection of Seasonal Sashimi (Change 3 kinds of sashimi to 5 kinds, additional charge of 1,500yen) Broiled Dish Broiled Black Cod Seasoned in Saikyo Miso Paste Roasted Chestnuts, Ginkgo Nuts, Boiled and Seasoned Vegetable with Bonito Flakes Turnip Fermented Sushi with Salmon Main Dish Fried White Fish, Taro, Wild Rice Stem, Leek and Ginger with Starchy Sauce or Assorted Tempura with Grated Radish, Ginger and Andes Salt Hot Dish Taro Dumpling Rice Dish Rice Cooked with White Maitake Mushroom or Steamed Rice Miso Soup and Japanese Pickles Dessert Assorted Seasonal Fruits with Jelly ¥8,000 The menu may change without prior notice. -

HEAT INACTIVATION of THIAMINASE in WHOLE FISH by R
August 1966 COMMERCIAL FISHERIES REVIEW 11 HEAT INACTIVATION OF THIAMINASE IN WHOLE FISH By R. H. Gnaedinger and R. A. Krzeczkowskil,c ABSTRACT The time required at various temperatures to inactivate all of the thiam inase in several species of whole fish was studied. Some effects of pH and enzyme concentra tion on the time-temperature inactivation were also determined. Whole raw fish were ground! sealed in spec~ally-constructed m etal cans, heated a t various tempera tures .for. varIOUS length.s <;>f tune! and analyzed for residual thiaminase a ct ivity. Re sul~ md.lcate that a m~un .um tune -tempe.rature of 5 minutes a t 1800 F. is required t<;> mac.tlvate all the .thl~mma s e of who.le hsh. Enzyme concentrations, pH, a nd pos slbly 011 c ontent of flsh mfluence the tune required to destroy thiaminase. INTRODUCTION The heating conditions employed b y commercial mink-food producers and mink ranchers ;0 destroy thiaminase in whole fish are empiri cal. The conditions are not based on predeter nined time-temperature relations for the thermal inactivation of this antimetabolite. A com mon practice, for example, is to cook the fish at 1800 -2000 F. for 15 minutes (Bor gstrom 1962). Most of the specific data available on the time -temperature r e la tion is found in various research publications dealing with the occurrence of thiamina s e in fish , or with studies on the chemistry of the enzyme. Deutsch and Hasler (1943) used 15 m i nutes at 100 0 C . -

Florida Spiny Lobster Glazing Florida Lobster
Seafood Safe Handling Tips Florida Spiny Lobster Glazing Florida Lobster Spiny lobster (Panulirus argus) is a crustacean related Frozen lobster is “glazed” with a thin coat of ice Purchase seafood last and keep it cold during the to crabs, shrimp, crayfish and the Spanish lobster. and packaged in plastic to protect the meat from trip home. Spiny lobster has numerous spines on the body, two dehydration and freezer burn. The net weight listed Keep raw and cooked seafood separate to prevent large hooked horns over the eyes, a pair of long, jointed on the packaging must be the “unglazed” weight of the bacterial cross-contamination. antennae and five pairs of walking legs but no claws. product. For weighing purposes, the product should The shell on the body and tail has mottled coloring of After handling raw seafood, thoroughly wash knives, be rinsed only long enough to remove the glaze. If the yellow, brown, orange and blue markings but it turns a cutting surfaces, sponges and hands with hot soapy glaze is excessive and you are charged lobster price for bright red-orange when the lobster is cooked. Florida water. excess ice, it is mislabeled. spiny lobster is commercially harvested off the southern tip of Florida and the Florida Keys. It is Mislabeling seafood is illegal. If you believe a seafood Buying and Storing Tips caught live using special traps set at depths of 6 to 300 product purchased from a seafood retail store or feet. Its diet consists of clams, snails, seaweed and small supermarket seafood counter is mislabeled, please Live lobster should have some leg movement marine organisms. -

FDA: "Glowing" Seafood?
FDA: "Glowing" Seafood? http://web.archive.org/web/20080225162926/http://vm.cfsan.fda.gov/~ea... U.S. Food and Drug Administration Seafood Products Research Center July 1998 "GLOWING" SEAFOOD? by Patricia N. Sado* Introduction Seafood that produces a bright, blue-green light in the dark could be a meal from outer space or haute cuisine in a science fiction novel. The U. S. Food and Drug Administration (FDA) has received many consumer complaints about various seafood products "glowing" in the dark. Some of these consumers called their local health departments, poison control centers, and their U.S. Senator because they thought they had been poisoned by radiation. These consumers said they had trouble convincing people that their seafood was emitting light. One consumer took his imitation crabmeat to a local television station. Unfortunately his seafood had dried out and did not glow for the television reporters. Several consumers said that it took them many weeks before they found phone numbers for various government agencies to make inquiries. Several consumers thought their "glowing" seafood was due to phosphorescing phytoplankton, or even fluorescence. The consumers' seafood products "glowing" in the dark were not due to radiation or to fluorescence, which requires an ultraviolet light to trigger the reaction. These seafood products exhibited luminescence due to the presence of certain bacteria that are capable of emitting light. Luminescence by bacteria is due to a chemical reaction catalyzed by luciferase, a protein similar to that found in fireflies. The reaction involves oxidation of a reduced flavin mononucleotide and a long chain aliphatic aldehyde by molecular oxygen to produce oxidized flavin plus fatty acid and light (5, 12). -

High-Pressure Processing for the Production of Added-Value Claw Meat from Edible Crab (Cancer Pagurus)
foods Article High-Pressure Processing for the Production of Added-Value Claw Meat from Edible Crab (Cancer pagurus) Federico Lian 1,2,* , Enrico De Conto 3, Vincenzo Del Grippo 1, Sabine M. Harrison 1 , John Fagan 4, James G. Lyng 1 and Nigel P. Brunton 1 1 UCD School of Agriculture and Food Science, University College Dublin, Belfield, D04 V1W8 Dublin, Ireland; [email protected] (V.D.G.); [email protected] (S.M.H.); [email protected] (J.G.L.); [email protected] (N.P.B.) 2 Nofima AS, Muninbakken 9-13, Breivika, P.O. Box 6122, NO-9291 Tromsø, Norway 3 Department of Agricultural, Food, Environmental and Animal Sciences, University of Udine, I-33100 Udine, Italy; [email protected] 4 Irish Sea Fisheries Board (Bord Iascaigh Mhara, BIM), Dún Laoghaire, A96 E5A0 Co. Dublin, Ireland; [email protected] * Correspondence: Federico.Lian@nofima.no; Tel.: +47-77629078 Abstract: High-pressure processing (HPP) in a large-scale industrial unit was explored as a means for producing added-value claw meat products from edible crab (Cancer pagurus). Quality attributes were comparatively evaluated on the meat extracted from pressurized (300 MPa/2 min, 300 MPa/4 min, 500 MPa/2 min) or cooked (92 ◦C/15 min) chelipeds (i.e., the limb bearing the claw), before and after a thermal in-pack pasteurization (F 10 = 10). Satisfactory meat detachment from the shell 90 was achieved due to HPP-induced cold protein denaturation. Compared to cooked or cooked– Citation: Lian, F.; De Conto, E.; pasteurized counterparts, pressurized claws showed significantly higher yield (p < 0.05), which was Del Grippo, V.; Harrison, S.M.; Fagan, possibly related to higher intra-myofibrillar water as evidenced by relaxometry data, together with J.; Lyng, J.G.; Brunton, N.P. -

City Island Lobster.062315.Indd
City Island Appetizers Lobster House Crab Corner City Island Cold Hot From Alaska From Maryland Favorites Jumbo Shrimp Cocktail (5) ......................... 16.95 Bu alo Wings (12) ...................................... 12.95 King Crab Legs ............................................. 60.95 Whole Blue Claw Crabs .............................. 29.95 Favorites Whole Lobster in Shell (cold) ..................... 22.95 Mozzarella Sticks (8) ................................... 11.95 Snow Crab Leg Clusters ............................... 32.95 served broiled or steamed or Baltimore Style Catch of the Day Scungilli Salad ............................................... 15.95 Coconut Shrimp ........................................... 16.95 (spicy w/Old Bay) also served w/garlic or red sauce. Twin Lobster Tail Platter ................................... 45.95 Calamari Salad ............................................. 14.95 Fried Calamari .............................................. 13.95 Jumbo Shrimp Scampi ....................................... 26.95 Halibut or Sword sh .......................................... 27.95 Served broiled or steamed .... Freshly Shucked Littleneck Fried Ravioli ................................................. 12.95 Jumbo Dungeness Crab Claw Clusters 33.95 Baked Scallops & Shrimp Combination .......... 42.95 Norwegian Salmon ............................................ 25.95 or Cherrystone Clams ...................(½ Doz.) 10.00 Tender Chicken Fingers ............................... 12.95 Super Crab Lovers Combination -

Appetizers & Seafood Classics Housemade Salads Live
HOUSEMADE SALADS Housemade Dressings: Cheddar Cheese | Honey Mustard APPETIZER PLATTERS Basil Vinaigrette | Blue Cheese | Olive Oil and Red Wine Vinegar HOT APPETIZER PLATTER Ranch | Creamy Garlic & Peppercorn | Avocado Ranch fat free Honey French with Sundried Tomato grilled shrimp | baked oysters Rockefeller baked oysters Chesapeake | fried calamari HOUSE SALAD, CAESAR SALAD, crab-stuffed mushrooms | drawn butter WEDGE OF LETTUCE 6.75 mustard sauce | marinara sauce MARYLAND SEAFOOD SALAD 50 blue crab | shrimp | scallops | fresh greens COLD APPETIZER PLATTER avocado ranch 15.5 chilled peel ’n eat shrimp | smoked salmon* SEARED AHI TUNA SALAD lump blue crab cocktail blackened Rare over spinach fresh oysters on the half-shell* romaine & Asian slaw mixture | oriental noodles mustard sauce | cocktail sauce wasabi peas | soy ginger vinaigrette 15 50 LIVE MAINE LOBSTER The American lobster, commonly known as the Maine lobster, thrives from the coast of Cape Hatteras to as far north as Nova Scotia. APPETIZERS & SEAFOOD CLASSICS At Chesapeake’s we carry several sizes of Maine lobster to please all appetites. Served with baked potato and your choice of one side. CRAB BISQUE Cup 4.75 Bowl 7.5 SMOKED TROUT 12.5 1 1/2 LB. LIVE MAINE LOBSTER steamed, drawn butter 38 GRILLED SHRIMP 12.5 Lobsters are available in larger sizes at MKT for each additional 1/2 lb MUSHROOMS STUFFED WITH CRAB 12.5 over the 1 1/2 lb. price. FRIED CALAMARI STUFFED MAINE LOBSTER marinara sauce | mustard sauce 12.5 Crab Imperial Lobster Price Plus 12 SHRIMP COCKTAIL cocktail sauce 14 STEAMED SEAFOOD FEAST live Maine lobster | mussels MARYLAND CRAB CAKE Maryland spiced shrimp | clams | oysters lump blue crab meat seasoned bread crumbs | tartar sauce 12.5 Lobster Price Plus 15 BLUE CRAB COCKTAIL 15.5 ALL LOBSTER SIZES SUBJECT TO AVAILABILITY KING CRAB COCKTAIL 22 ONION RING PLATTER 10 SANDWICHES 12.5 SMOKED SALMON* Served with choice of one side. -

Chef Hopes to Make Carp a Popular Dish
02/28/2011 Chef Hopes To Make Carp A Popular Di… Chef Hopes To Make Carp A Popular Dish January 8, 2010 text size A A A Web Resources Asian carp may be a problem in the Great Lakes, but in Louisiana they Techniques to cook Asian carp are renaming it silver fin and promoting it as a delicacy. Chef Philippe Recipes for silverfin carp Parola says the Asian carp's texture is a cross between scallop and crabmeat, adding its bones can be removed easily by steaming. Copyright © 2010 National Public Radio®. For personal, noncommercial use only. See Terms of Use. For other uses, prior permission required. MELISSA BLOCK, host: The Louisiana Department of Wildlife and Fisheries has one approach to the carp conundrum: If you can't beat them, eat them. Next week, the state is in fact launching a campaign celebrating the gastro potential of the carp and giving it a new name, the silver fin. But how do you eat them? Well, that's where our next guest comes in handy. Chef Philippe Parola is working with the state to help people figure out how to cook the carp. And he joins us from Baton Rouge. Chef Parola, what does carp taste like? How would you describe it? Mr. PHILIPPE PAROLA (Chef): Well, number one, carp is kind of misleading on that name because the carp that we know in Louisiana, the common carp, they are bottom feeder, and they taste good, but not as good as the silver carp, which we tried to rename it as a silver fin. -
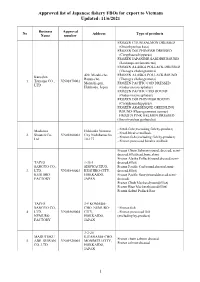
Approved List of Japanese Fishery Fbos for Export to Vietnam Updated: 11/6/2021
Approved list of Japanese fishery FBOs for export to Vietnam Updated: 11/6/2021 Business Approval No Address Type of products Name number FROZEN CHUM SALMON DRESSED (Oncorhynchus keta) FROZEN DOLPHINFISH DRESSED (Coryphaena hippurus) FROZEN JAPANESE SARDINE ROUND (Sardinops melanostictus) FROZEN ALASKA POLLACK DRESSED (Theragra chalcogramma) 420, Misaki-cho, FROZEN ALASKA POLLACK ROUND Kaneshin Rausu-cho, (Theragra chalcogramma) 1. Tsuyama CO., VN01870001 Menashi-gun, FROZEN PACIFIC COD DRESSED LTD Hokkaido, Japan (Gadus macrocephalus) FROZEN PACIFIC COD ROUND (Gadus macrocephalus) FROZEN DOLPHIN FISH ROUND (Coryphaena hippurus) FROZEN ARABESQUE GREENLING ROUND (Pleurogrammus azonus) FROZEN PINK SALMON DRESSED (Oncorhynchus gorbuscha) - Fresh fish (excluding fish by-product) Maekawa Hokkaido Nemuro - Fresh bivalve mollusk. 2. Shouten Co., VN01860002 City Nishihamacho - Frozen fish (excluding fish by-product) Ltd 10-177 - Frozen processed bivalve mollusk Frozen Chum Salmon (round, dressed, semi- dressed,fillet,head,bone,skin) Frozen Alaska Pollack(round,dressed,semi- TAIYO 1-35-1 dressed,fillet) SANGYO CO., SHOWACHUO, Frozen Pacific Cod(round,dressed,semi- 3. LTD. VN01840003 KUSHIRO-CITY, dressed,fillet) KUSHIRO HOKKAIDO, Frozen Pacific Saury(round,dressed,semi- FACTORY JAPAN dressed) Frozen Chub Mackerel(round,fillet) Frozen Blue Mackerel(round,fillet) Frozen Salted Pollack Roe TAIYO 3-9 KOMABA- SANGYO CO., CHO, NEMURO- - Frozen fish 4. LTD. VN01860004 CITY, - Frozen processed fish NEMURO HOKKAIDO, (excluding by-product) FACTORY JAPAN -

Seafood Seafood
MONDAYS 410.877.2900 $2 OFF www.theseafoodstop.com PLATTERS PLATTERS FEATURED FAVORITES Platters include choice of two specialty sides: FISH THE WEB! French Fries, Cole Slaw, Deviled Eggs, Potato Salad, Homemade Cheesy Crab Dip . Pound 14 .99 Tomato-Cucumber Salad (seasonal), Hush Puppies, Macaroni Shrimp Salad, Broccoli Salad Crab Pretzel . 7 .99 Stuffed Shrimp . Each 3 .99 The Captain’s Seafood . 26 .99 The A generous portion of haddock, two large sea scallops, two jumbo gulf shrimp, and a half Homemade Crab Balls . Dozen 19 .99 pound jumbo lump crab cake, fried or broiled . The Captain’s Choice! Clam Strips . 1 .99 Big Jimmie . 27 .99 Seafood Hush Puppies . (12) 2 .99 A half pound jumbo lump crab cake broiled, one fillet of orange roughy, fried or broiled, and a half pound of large steamed shrimp . Crab Eggs . Each 1 .49 . Dozen 15 .99 Stop Jumbo Lump Crab cake (Half Pounder!) . 20 .99 Deviled Eggs . Each 0 .69 . Dozen 7 .99 HOMEMADE from fresh jumbo lump crabmeat handpicked and broiled . Fried Shrimp . (3) 3 .75 . (12) 12 .99 Crab Imperial . 17 .99 French Fries . SM 1 .50 . .LG 2 .50 . XL 3 .99 Jumbo lump crabmeat blended with a creamy mixture of spices and peppers broiled and served in a scallop shell . Seafood Bucket . 25 .99 Two dozen steamed clams, two pounds of steamed mussels, one pound of large steamed Large Scallops . 19 .99 shrimp with cocktail sauce and melted butter . Eight Large sea scallops cooked to perfection, batter fried or broiled . Fried Shrimp . 13 .99 Six, single padded gulf shrimp, deep fried . -

DINNER RAW BAR HANDSHUCKED OYSTERS $M/P House Cocktail Sauce, Champagne Mignonette, Preserved Lemon
LUNCH RAW BAR SCARLET SEAFOOD TOWER $165 3 Tiers of Alaskan King Crab Legs, East & West Coast Oysters, Chilled Maine Lobster, Jumbo Shrimp, Mussels, Jumbo Lump Crab Meat, Ceviche de Pescado. House Mignonette, Cocktail Sauce, Lemons, Horseradish Serves Four to Six Guests JUMBO SHRIMP COCKTAIL $22 House Cocktail Sauce, Lemon CHILLED ALASKAN SNOW CRAB LEGS $MK Clarified Butter, Preserved Lemon OYSTERS ON THE HALF SHELL House Cocktail Sauce, Champagne Mignonette, Preserved Lemon. EAST COAST Daily Selection Half Dozen $18 Dozen $35. WEST COAST Daily Selection Half Dozen $22 Dozen $42 CHILLED 1 1/2 LB MAINE LOBSTER $MK Dijonaisse, Clarified Butter, Preserved Lemon STARTERS ROASTED KABOCHA SQUASH BISQUE $10 Truffle Maple Cream SPICY HOMEMADE GUACAMOLE $13 Serrano Pepper, Avocado, Onion, Cilantro, Corn Chips. Add Lobster $9 MARGARITA FLATBREAD $15 San Marzano Tomato Sauce, Fresh Mozzarella, Fresh Basil LOBSTER FLATBREAD $24 Grilled Crust with Fontina Cheese, Buttered Lobster, Tomatoes, Garlic, Basil, Sweet Drop Peppers CRISPY POINT JUDITH CALAMARI $17 Flash-Fried, Sweet Drop Peppers, Scallions, Served with Lime Chipotle Aioli BURRATA HEIRLOOM TOMATO SALAD $14 Locally Sourced Burrata, Path Valley Farm Heirloom Tomatoes, Baby Arugula, Red Onion, Basil Vinaigrette WEDGE SALAD $11 Baby Iceberg Lettuce, Tomatoes Tossed in Shallots- Sherry Vinaigrette, Bacon, Chives, Bleu-Cheese Crumbles, Bleu-Cheese Dressing BURRATA BEETS SALAD $14 Locally Sourced Burrata, Path Valley Farms Heirloom Beets, Baby Arugula, Red Onion, Basil Oil, Lavender Vinaigrette HANDHELDS -

Preparing Blue Crab: a Seafood Delicacy1 Donald E
SGEF120 Preparing Blue Crab: A Seafood Delicacy1 Donald E. Sweat2 Choosing Pay $15 per dozen 1 crab weighs 1/3 pound Like its crustacean cousins, shrimp and spiny lobsters, the 1 dozen crabs weigh 4 pounds blue crab ranks high on the list of seafood delicacies in Florida. Although blue crab meat is available year -round in the pasteurized form, live crabs are seasonal and much more plentiful during the warm-water months of the year. Fresh or pasteurized cooked crab meat is usually available for purchase as lump, flake, or claw meat. Lump meat consists of whole lumps from the large body muscles which operate the swimming legs. Flake meat consists of small pieces of white meat from the body. Claw meat consists of a brownish-tinged meat from the claws. Blue crabs in seafood markets usually are sold by the dozen. An average blue crab weighs about 1/3 pound, but the edible portion is quite low. An experienced crab picker can produce about 21/4 ounces of meat from each pound of live blue crabs. This is just about a 14 percent yield. The actual yield depends on the size of the individual crab and experi- ence of the crab picker. The consumer is probably better off to purchase the crab meat already prepared unless the picking is incorporated into a “crab boil” or “picnic type” activity. You can estimate a price comparison between fresh andpasteurized meat by studying the following example: Donald E. Sweat, UF/IFAS Extension Marine Agent, demonstrates the preparation of blue crabs. Credits: UF/IFAS 1.