Diversity and Abundance of Zooplankton in River Aswa in Uganda
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wetlands of the Nile Basin the Many Eco for Their Liveli This Chapt Distribution, Functions and Contribution to Contribution Livelihoods They Provide
important role particular imp into wetlands budget (Sutch 11 in the Blue N icantly 1110difi Wetlands of the Nile Basin the many eco for their liveli This chapt Distribution, functions and contribution to contribution livelihoods they provide. activities, ane rainfall (i.e. 1 Lisa-Maria Rebelo and Matthew P McCartney climate chan: food securit; currently eX' arc under tb Key messages water resour support • Wetlands occur extensively across the Nile Basin and support the livelihoods ofmillions of related ;;ervi people. Despite their importance, there are big gaps in the knowledge about the current better evalu: status of these ecosystems, and how populations in the Nile use them. A better understand systematic I ing is needed on the ecosystem services provided by the difl:erent types of wetlands in the provide. Nile, and how these contribute to local livelihoods. • While many ofthe Nile's wetlands arc inextricably linked to agricultural production systems the basis for making decisions on the extent to which, and how, wetlands can be sustainably used for agriculture is weak. The Nile I: • Due to these infi)fl11atio!1 gaps, the future contribution of wetlands to agriculture is poorly the basin ( understood, and wetlands are otten overlooked in the Nile Basin discourse on water and both the E agriculture. While there is great potential for the further development of agriculture and marsh, fen, fisheries, in particular in the wetlands of Sudan and Ethiopia, at the same time many that is stat wetlands in the basin are threatened by poor management practices and populations. which at \, In order to ensure that the future use of wetlands for agriculture will result in net benefits (i.e. -

Molecular Systematics of Freshwater Diaptomid Species of the Genus Neodiaptomus from Andaman Islands, India
www.genaqua.org ISSN 2459-1831 Genetics of Aquatic Organisms 2: 13-22 (2018) DOI: 10.4194/2459-1831-v2_1_03 RESEARCH PAPER Molecular Systematics of Freshwater Diaptomid Species of the Genus Neodiaptomus from Andaman Islands, India B. Dilshad Begum1, G. Dharani2, K. Altaff3,* 1 Justice Basheer Ahmed Sayeed College for Women, P. G. & Research Department of Zoology, Teynampet, Chennai - 600 018, India. 2 Ministry of Earth Sciences, Earth System Science Organization, National Institute of Ocean Technology, Chennai - 600 100, India. 3 AMET University, Department of Marine Biotechnology, Chennai - 603112, India. * Corresponding Author: Tel.: +9444108110; Received 10 April 2018 E-mail: [email protected] Accepted 29 July 2018 Abstract Calanoid copepods belonging to the family Diaptomidae occur commonly and abundantly in different types of freshwater environment. Based on morphological taxonomic key characters 48 diaptomid species belonging to 13 genera were reported from India. Taxonomic discrimination of many species of these genera is difficult due to their high morphological similarities and minute differences in key characters. In the present study two species of the genus, Neodiaptomus, N. meggiti and N. schmackeri from Andaman Islands were examined based on morphological and molecular characters which showed low variation in morphology and differences in their distributions. The morphological taxonomy of Copepoda with genetic analysis has shown complementing values in understanding the genetic variation and phylogeny of the contemporary populations. In this study, a molecular phylogenetic analysis of N. meggiti and N. schmackeri is performed on the basis of mitochondrial Cytochrome c oxidase subunit I (COI) gene. The mtDNA COI sequence of N. meggiti and N. -

Anatomy of the Nile Following the Twists and Turns of the World's Longest River
VideoMedia Spotlight Anatomy of the Nile Following the twists and turns of the world's longest river For the complete video with media resources, visit: http://education.nationalgeographic.org/media/anatomy-nile/ Funder The Nile River has provided fertile land, transportation, food, and freshwater to Egypt for more than 5,000 years. Today, 95% of Egypt’s population continues to live along its banks. Where does the Nile begin? Where does it end? Watch this video, from Nat Geo WILD’s “Destination Wild” series, to find out. For an even deeper look at the Nile, use our vocabulary list and explore our “geo-tour” of the Nile to understand the geography of the river and answer the questions in the Questions tab. Questions Where is the source, or headwaters, of the Nile River? The streams of Rwanda’s Nyungwe Forest are probably the most remote sources of the Nile. The snow-capped peaks of the Rwenzori Mountains are another one of the remote sources of the Nile. The Rwenzori Mountains, sometimes nicknamed the “Mountains of the Moon,” straddle the border between the Democratic Republic of the Congo and Uganda. Many geographers also consider Lake Victoria, the largest lake in Africa, to be a source of the Nile. The most significant outflow from Lake Victoria, winding northward through Uganda, is called the “Victoria Nile.” Can you find a waterfall on the Nile River? As it twists more than 6,500 kilometers (4,200 miles) through Africa, the Nile has dozens of small and large waterfalls. The most significant waterfall on the Nile is probably Murchison Falls, Uganda. -

Ctenodiaptomus) Praedictus Sulawensis Alekseev & Vaillant, 2013 (Hexanauplia, Copepoda, Calanoida, Diaptomidae) in the Philippines (Luzon Island
PRIMARY RESEARCH PAPER | Philippine Journal of Systematic Biology New record of Phyllodiaptomus (Ctenodiaptomus) praedictus sulawensis Alekseev & Vaillant, 2013 (Hexanauplia, Copepoda, Calanoida, Diaptomidae) in the Philippines (Luzon Island) Shea Kathleen P. Guinto1, Justine Val Jade B. Lacaba2, John Kenneth V. Cuballes2, Aezrile A. Igancio2, Eric Zeus C. Rizo3, Henri J. Dumont3,4, Bo-Ping Han3 & Rey Donne S. Papa1,2,5* ABSTRACT A study originally intended to update the taxonomy and distribution of calanoid copepods in selected freshwater ecosystems of Central Luzon has led to the discovery of a new record of Phyllodiaptomus Kiefer, 1936 in Candaba Swamp, Pampanga. Since 1979, the only calanoid copepods recorded from this area included Filipinodiaptomus insulanus (Wright S., 1928) and Tropodiaptomus australis Kiefer, 1936. Later studies on calanoid copepods in the region have since been non-existent. Analyses of pertinent key morphological characters revealed that the specimens at hand belonged to Phyllodiaptomus (Ctenodiaptomus) praedictus sulawensis Alekseev & Vaillant, 2013, a freshwater diaptomid calanoid copepod subspecies discovered and known to be endemic only in Indonesia. Provided in this paper are baseline information on the morphological characters of the Philippine members of the subspecies accompanied by line drawings as well as a comparison between the recorded morphological data presented by Alekseev, Haffner, Vaillant & Yusoff (2013) and the current dataset to support the identification of the specimen. The discovery of P. (C.) praedictus sulawensis in the Philippines, which was thought to be endemic in Indonesia, presents a new record of this species in the country and the first such record outside of its country of origin. KEYWORDS: Candaba Swamp, Copepod, Indonesia, Inland Waters, Limnology, Thailand INTRODUCTION eastern, and eastern Asia with majority found in Thailand and India. -

Sri Lanka Freshwater Namely the Cyclopoija Tfree Living and Parasite, Calanoida and Harpa::Ticoida
C. H. FERNANDO 53 Fig. 171 (contd: from page 52) Sphaericus for which an Ontario specimen was used. I have illustrated some of the head shields of Chydoridae. The study of Clackceran remains so commonly found in samples emLbles indonti:fication ,,f species which have been in the habita'~ besides those act_ive stages when the samples was collected. Males of Cladocera are rare but they are of considerable value in reaching accurate diagnoses of species. I have illustrated the few males I have .found in the samples. A more careful study of all the specimens will certainly give males of most s1)ecies sin00 ·bhe collections were made throughout the year. REFERRENCES APSTEIN, C. (1907)-Das plancton in Colombo see auf Ceylon. Zool. Jb. (Syst.) 25 :201-244. l\,J>STEJN, C. (1910)-Das plancton des Gregory see auf Ceylon. Zool. Jb. (Syst.) 29 : 661-680. BAIRD, W. (1849)-Thenaturalhistory oftheBritishEntomostraca. Ray Soc. Lond. 364pp. BAR, G.(1924)-UberCiadoceren von derlnsel Ceylon (Fauna etAnatomia Ceylonica No.14) Jena. Z.Naturw. 60: 83-125. BEHNING, A. L. (1941)-(Kladotsera Kavkasa) Cladocera of the Caucasus (In Rusian) Tbilisi, Gzushedgiz. 383 pp. BIRABEN, M. (1939)-Los Cladoceros d'Lafamilie "Chydoridae". Physis. (Rev. Soc. Argentina Cien. Natur.) 17, 651-671 BRADY, G. S. (1886)-Notes on Entomostraca collected by Mr. A. Haly in Ceylon. Linn. Soc. Jour. Lond. (Zool.) 10: 293-317. BRANDLOVA, J., BRANDL. Z., and FERNANDO, C. H. (1972)-The Cladoceraof Ontariowithremarksonsomespecie distribution. Can. J. Zool. 50 : 1373-1403. BREHM, V. (1909)-Uber die microfauna chinesicher and sudasiatischer susswassbickers. Arch. Hydrobiol. 4, 207-224. -

Assessing the Bujagali Hydropower Project in Uganda
Modern Approaches in Oceanography and Petrochemical Sciences DOI: 10.32474/MAOPS.2019.02.000141 ISSN: 2637-6652 Research Article Assessing the Bujagali Hydropower Project in Uganda George Kimbowa1 and Khaldoon A Mourad2* 1Busitema University, Uganda 2Center for Middle Eastern Studies, Lund, Sweden *Corresponding author: Khaldoon A Mourad, Center for Middle Eastern Studies, Lund, Sweden Received: January 21, 2019 Published: January 29, 2019 Abstract The development of great dams and hydropower plants increases power supply and access. However, the process is considered a threat to livelihoods, ecosystem and biodiversity because in most cases it brings about human displacement and natural resources degradation. This paper seeks to assess the development of the Bujagali Hydropower Plant in Uganda (BHP) and its compliance with IWRM principles based on water knowledges, societal values, and inter-disciplinary approach. The paper develops a set of strategic interventions for the dam and the BHP based on SWOT analysis, XLRM framework, Multi-sectoral and interdisciplinary development approach, and sustainable management. These measures are deemed socially and ecologically acceptable by all stakeholders including the cultural and historical institutions, societal actor groups, including mega-hydraulic bureaucracies, the private sectors and national politicians. The results show that project developers should always carry out Environment and Social Impact Assessments (ESIA); develop timely ‘Resettlement Action Plan’; carry out informed consultation and participation; promote transparency; and communicate project’s risks, potential impacts and probable mitigation actions to attain sustainability. The paper proposes some policy interventions to be implemented along the project’s lifetime. Furthermore, it presents a sustainable development plan for such projects based on the IWRM principles. -

Bujagali Final Report
INDEPENDENT REVIEW PANEL COMPLIANCE REVIEW REPORT ON THE BUJAGALI HYDROPOWER AND INTERCONNECTION PROJECTS June 20, 2008 1 ACKNOWLEDGEMENTS The IRM Compliance Review Panel could not have undertaken and completed this report without the generous assistance of many people in Uganda and at the African Development Bank. It wishes to express its appreciation to all of them for their cooperation and support during the compliance review of the Bujagali Hydropower and Interconnection projects. The Panel thanks the Requesters and the many individuals from civil society and the communities that it met in the Project areas and in Kampala for their assistance. It also appreciates the willingness of the representatives of the Government of Uganda and the projects’ sponsors to meet with the Panel and provide it with information during its visit to Uganda. The Panel acknowledges all the help provided by the Resident Representative of the African Development Bank in Uganda and his staff and the willing cooperation it has received from the Bank’s Management and staff in Tunis. The Panel appreciates the generous cooperation of the World Bank Inspection Panel which conducted its own review of the “UGANDA: Private Power Generation Project”. The Compliance Review Panel and the World Bank Inspection Panel coordinated their field investigations of the Bujagali projects and shared consultants and technical information during this investigation in order to enhance the efficiency and cost effectiveness of each of their investigations. While this collaboration between the Panel and the World Bank Inspection Panel worked to the mutual benefit of both parties, each Panel focused its compliance review on its own Bank’s policies and procedures and each Panel has made its own independent judgments about the compliance of its Management and staff with its Bank’s policies and procedures. -

FY 2019/20 Vote:589 Bulambuli District
LG Approved Workplan Vote:589 Bulambuli District FY 2019/20 Foreword the draft performance contract form B in a decentralized environment provides a clear logical link between the 5year development plan that bears a the vision empowered and prosperous people of Bulambuli with a middle income status of $3000 per capita income by 2020.The contact equally focuses on fulfilling the district mission to provide quality and coordinated services focusing on national and local priorities for transformation and to enable the people into a prosperous society of Bulambuli district accessing quality services by 2040 I wish to remind all stakeholders that the struggle for the development of Bulambuli continues,much is still needed to be done thus your unreserved efforts are all called for,I appeal to all political,technical staff to accord the draft performance contract form B it needs to make the dream of improved quality of life of the people of Bulambuli come true. FOR GOD AND MY COUNTRY Wadada Lawrence Generated on 19/07/2019 02:16 1 LG Approved Workplan Vote:589 Bulambuli District FY 2019/20 SECTION A: Workplans for HLG Workplan 1a Administration Quarterly Workplan Outputs for FY 2019/20 Ushs Thousands Approved Budget Expenditure and Annual Planned Quarter 1 Quarter 2 Quarter 3 Quarter 4 and Outputs for Outputs by end Spending and Planned Spending Planned Planned Spending Planned Spending FY 2018/19 March for FY Outputs FY and Outputs Spending and and Outputs and Outputs 2018/19 2019/20 Outputs Programme: 13 81 District and Urban Administration Class Of OutPut: Higher LG Services Output: 13 81 01Operation of the Administration Department Non Standard Outputs: Coordination, - Coordination, -Health centres -Health centres - Headquarter - Headquarter - 26 sub counties supervision, supervision, monitored and all monitored and all departments departments and 3 Town monitoring and monitoring & staff on duty. -
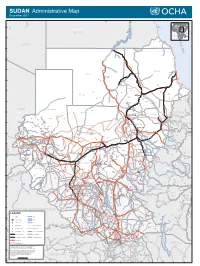
SUDAN Administrative Map December 2011
SUDAN Administrative Map December 2011 Faris IQLIT Ezbet Dush Ezbet Maks el-Qibli Ibrim DARAW KOM OMBO Al Hawwari Al-Kufrah Nagel-Gulab ASWAN At Tallab 24°N EGYPT 23°N R E LIBYA Halaib D S 22°N SUDAN ADMINISTRATED BY EGYPT Wadi Halfa E A b 'i Di d a i d a W 21°N 20°N Kho r A bu Sun t ut a RED SEA a b r A r o Porth Sudan NORTH Abu Hamad K Dongola Suakin ur Qirwid m i A ad 19°N W Bauda Karima Rauai Taris Tok ar e il Ehna N r e iv R RIVER NILE Ri ver Nile Desert De Bayouda Barbar Odwan 18°N Ed Debba K El Baraq Mib h o r Adara Wa B a r d a i Hashmet Atbara ka E Karora l Atateb Zalat Al Ma' M Idd Rakhami u Abu Tabari g a Balak d a Mahmimet m Ed Damer Barqa Gereis Mebaa Qawz Dar Al Humr Togar El Hosh Al Mahmia Alghiena Qalat Garatit Hishkib Afchewa Seilit Hasta Maya Diferaya Agra 17°N Anker alik M El Ishab El Hosh di El Madkurab Wa Mariet Umm Hishan Qalat Kwolala Shendi Nakfa a r a b t Maket A r a W w a o d H i i A d w a a Abdullah Islandti W b Kirteit m Afabet a NORTH DARFUR d CHAD a Zalat Wad Tandub ug M l E i W 16°N d Halhal Jimal Wad Bilal a a d W i A l H Aroma ERITREA Keren KHARTOUM a w a KASSALA d KHARTOUM Hagaz G Sebderat Bahia a Akordat s h Shegeg Karo Kassala Furawiya Wakhaim Surgi Bamina New Halfa Muzbat El Masid a m a g Barentu Kornoi u Malha Haikota F di Teseney Tina Um Baru El Mieiliq 15°N Wa Khashm El Girba Abu Quta Abu Ushar Tandubayah Miski Meheiriba EL GEZIRA Sigiba Rufa'ah Anka El Hasahisa Girgira NORTH KORDOFAN Ana Bagi Baashim/tina Dankud Lukka Kaidaba Falankei Abdel Shakur Um Sidir Wad Medani Sodiri Shuwak Badime Kulbus -

The Politics of the Nile Basin
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Wits Institutional Repository on DSPACE THE POLITICS OF THE NILE BASIN ELIAS ASHEBIR Supervisor:- Larry Benjamin A Dissertation Submitted to the Department of International Relations, at the University of the WitWatersRand, in Partial Fulfillment of the Requirements for Obtaining the Degree of Master of Arts in Hydropotitics Studies Johannesburg 2009 DECLARATION I hereby declare that this dissertation is my own unaided and has not been submitted to any other University for any other degree. Elias Ashebir May 2009 2 TABLE OF CONTENTS Acknowledgment.............................. VI Abstract ................................... VII Introduction................................ VIII Chapter I A Brief Survey of the Nile Basin 1. General overview 1-3 2. Exploration of the Nile 3. Geographical & Hydrological Feature of the Nile Basin 3-4 3.1 The Blue Nile 4 3.2 The White Nile 4-9 Chapter II The Nile Riparian Countries & Future Challenges 1. Subsystems of the Nile Basin 10 1.1 The White Nile Subsystem 11 1.2 The Abbay (Blue Nile) Subsystem 11-12 1.3 The Tekeze (Atbara) Subsystem 12 1.4 The Baro-Akobo (Sobat) Subsystem 12-13 2. General Descriptions of the Nile Riparian Countries 2.1 Upper Riparian Countries of the Nile Basin a) Ethiopia 14-24 b) Eritrea 24-26 c) Kenya 27-32 2.2 The Equatorial upper riparian countries a) Tanzania 32-37 b) Uganda 37-41 c) Democratic Republic of Congo 42-46 3 d) Rwanda 47-50 e) Burundi 50-53 2.3 The Lower riparian countries a) Egypt 53-57 b) Sudan 57-62 Chapter III Legal aspects of the use of the Nile waters 1. -

USAID/Uganda's District-Based Technical Assistance (DBTA)
ANNEX A. STATEMENT OF WORK STATEMENT OF WORK FOR EVALUATION OF USAID/UGANDA’S DISTRICT-BASED TECHNICAL ASSISTANCE (DBTA) PROJECTS, STRENGTHENING TUBERCULOSIS AND HIV/AIDS RESPONSES (STAR) PROJECTS IN EAST, EAST-CENTRAL, AND SOUTH-WEST UGANDA INTRODUCTION The STAR projects in East, East-Central, and South-West Uganda were the first in USAID/Uganda’s District Based Technical Assistance (DBTA) model featuring regional focus on improving accessibility, quality, and availability of integrated health service delivery as well as health financing and management. The STAR program is implemented by Management Sciences for Health (MSH) in East Uganda, by John Snow International (JSI) in East-Central Uganda, and by Elizabeth Glaser Pediatric AIDS Foundation (EGPAF) in South-West Uganda, covering thirty- four districts in total. Working closely with the Ministry of Health and through district health management teams (DHMTs), district councils, health facilities, and communities, the projects’ goal is to increase access to, coverage of, and utilization of quality comprehensive HIV/AIDS and TB prevention, care, and treatment services within district health facilities and their respective communities. This will be achieved through the following objectives: (a) strengthening decentralized HIV/AIDS and TB service delivery systems; (b) improving the quality and efficiency of HIV/AIDS and TB service delivery within health facilities; (c) strengthening networks and referrals systems to improve access to, coverage of, and use of HIV/AIDS and TB services; and (d) increasing demand for comprehensive HIV/AIDS and TB prevention, care, and treatment services. All three STAR projects are designed to strengthen systems at the decentralized level to facilitate improved delivery and uptake of HIV/AIDS and TB services, including district-led performance reviews to help identify coverage and service gaps. -

Building Resilience in Uganda's Watersheds
Environmental and Social Management Framework (ESMF) Project Title: Strengthening the Adaptive Capacity and Resilience of Communities in Uganda's watersheds - Awoja Catchment (SACRiAC) Country(ies): Uganda GEF Project ID: 10203 GEF Agency(ies): AfDB GEF Agency Project ID: Project Executing Entity(s): Ministry of Water and Submission Date: Environment GEF Focal Area (s): Climate Change Expected Implementation Start Expected Completion Date Name of Parent Program [if applicable] Parent Program ID: Summary The Government of the Republic of Uganda has received financing from the Global Environment Facility (GEF) for the development of the “Strengthening the Adaptive Capacity and Resilience of Communities in Uganda's watersheds” project. The project aims to strengthen resilience of approximately half a million vulnerable people to the impacts of climate change, through adaptation technology transfer (strategic objective 1) and climate mainstreaming (strategic objective 2). This project has four components, namely: 1) Climate resilient infrastructure implemented for enhanced livelihoods, 2) Strengthened capacity of communities and institutions for climate resilient planning in four watersheds, 3) Climate information integrated into development plans & early warning systems, and 4) Monitoring and Evaluation (M&E) and Adaptation Learning. The project will be implemented in the sub-catchments of Komirya, Sironko, Simu-sisi, Muyembe and Sipi (in the districts of Bukedea, Sironko, Bulambuli and Kapchorwa) within the Awoja catchment. The purpose of this ESMF is to set out a unified process for assessing and managing all environmental and social safeguard issues for subprojects from preparation, through appraisal and approval, to implementation. The ESMF gives information on how to address adverse environmental and social impacts of components of the project and will be applied in sub-projects.