Levobunolol Compared with Timolol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intraocular Pressure Rise After Phacoemulsification Prophylactic
British Journal of Ophthalmology 1995; 79: 809-813 809 Intraocular pressure rise after phacoemulsification Br J Ophthalmol: first published as 10.1136/bjo.79.9.809 on 1 September 1995. Downloaded from with posterior chamber lens implantation: effect of prophylactic medication, wound closure, and surgeon's experience Thomas G Bomer, Wolf-Dietrich A Lagreze, Jens Funk Abstract pressure rises usually occur between 6 and 8 Aims-A prospective clinical trial was hours after surgery.6 carried out to evaluate the effect of Various antiglaucomatous agents have been prophylactic medication, the technique of used to prevent the intraocular pressure rise wound closure, and the surgeon's experi- after cataract extraction. Oral acetazolamide15 16 ence on the intraocular pressure rise after and topical timolol'6-19 lowered the pressure cataract extraction. rise in the early period after intracapsular and Methods-In 100 eyes, the intraocular extracapsular cataract extraction. Levobunolol pressure was measured before as well as proved to be superior to timolol 4-7 hours 2-4, 5-7, and 22-24 hours after phaco- after extracapsular cataract extraction.20 Apra- emulsification and posterior chamber lens clonidine lowered the intraocular pressure rise implantation. Each of 25 patients received after uncomplicated phacoemulsification21 and either 1% topical apraclonidine, 0.5%/o extracapsular cataract extraction22 23 when topical levobunolol, 500 mg oral acetazo- given 30 minutes to 1 hour before surgery, lamide, or placebo. Forty four eyes were whereas immediate postoperative treatment operated with sclerocorneal sutureless with apraclonidine was ineffective.22 24 Miotics tunnel and 56 eyes with corneoscleral are frequently used to promote miosis and incision and suture. -

Brimonidine Tartrate; Brinzolamide
Contains Nonbinding Recommendations Draft Guidance on Brimonidine Tartrate ; Brinzolamide This draft guidance, when finalized, will represent the current thinking of the Food and Drug Administration (FDA, or the Agency) on this topic. It does not establish any rights for any person and is not binding on FDA or the public. You can use an alternative approach if it satisfies the requirements of the applicable statutes and regulations. To discuss an alternative approach, contact the Office of Generic Drugs. Active Ingredient: Brimonidine tartrate; Brinzolamide Dosage Form; Route: Suspension/drops; ophthalmic Strength: 0.2%; 1% Recommended Studies: One study Type of study: Bioequivalence (BE) study with clinical endpoint Design: Randomized (1:1), double-masked, parallel, two-arm, in vivo Strength: 0.2%; 1% Subjects: Males and females with chronic open angle glaucoma or ocular hypertension in both eyes. Additional comments: Specific recommendations are provided below. ______________________________________________________________________________ Analytes to measure (in appropriate biological fluid): Not applicable Bioequivalence based on (95% CI): Clinical endpoint Additional comments regarding the BE study with clinical endpoint: 1. The Office of Generic Drugs (OGD) recommends conducting a BE study with a clinical endpoint in the treatment of open angle glaucoma and ocular hypertension comparing the test product to the reference listed drug (RLD), each applied as one drop in both eyes three times daily at approximately 8:00 a.m., 4:00 p.m., and 10:00 p.m. for 42 days (6 weeks). 2. Inclusion criteria (the sponsor may add additional criteria): a. Male or nonpregnant females aged at least 18 years with chronic open angle glaucoma or ocular hypertension in both eyes b. -

Table 1. Glaucoma Medications: Mechanisms, Dosing and Precautions Brand Generic Mechanism of Action Dosage/Avg
OPTOMETRIC STUDY CENTER Table 1. Glaucoma Medications: Mechanisms, Dosing and Precautions Brand Generic Mechanism of Action Dosage/Avg. % Product Sizes Side Effects Warnings Reduction CHOLINERGIC AGENTS Direct Pilocarpine (generic) Pilocarpine 1%, 2%, 4% Increases trabecular outflow BID-QID/15-25% 15ml Headache, blurred vision, myopia, retinal detachment, bronchiole constriction, Angle closure, shortness of breath, retinal narrowing of angle detachment Indirect Phospholine Iodide (Pfizer) Echothiophate iodide 0.125% Increases trabecular outflow QD-BID/15-25% 5ml Same as above plus cataractogenic iris cysts in children, pupillary block, Same as above, plus avoid prior to any increased paralysis with succinylcholine general anesthetic procedure ALPHA-2 AGONISTS Alphagan P (Allergan) Brimonidine tartrate 0.1%, 0.15% with Purite Decreases aqueous production, increases BID-TID/up to 26% 5ml, 10ml, 15ml Dry mouth, hypotension, bradycardia, follicular conjunctivitis, ocular irritation, Monitor for shortness of breath, dizziness, preservative uveoscleral outflow pruritus, dermatitis, conjunctival blanching, eyelid retraction, mydriasis, drug ocular redness and itching, fatigue allergy Brimonidine tartrate Brimonidine tartrate 0.15%, 0.2% Same as above Same as above 5ml, 10ml Same as above Same as above (generic) Iopidine (Novartis) Apraclonidine 0.5% Decreases aqueous production BID-TID/up to 25% 5ml, 10ml Same as above but higher drug allergy (40%) Same as above BETA-BLOCKERS Non-selective Betagan (Allergan) Levobunolol 0.25%, 0.5% Decreases -

Fluid Ophthalmic Composition Based on Lipid Microparticles Containing at Least One Active Principle
Europaisches Patentamt J European Patent Office Office europden des brevets (11) Publication number : 0 437 368 A1 EUROPEAN PATENT APPLICATION (21) Application number: 91300181.4 ® int. ci.5 : A61K 9/06, A61K 9/16 @ Date of filing : 10.01.91 © Priority : 12.01.90 FR 9000340 (72) Inventor : Rozier, Annouk 23 Bd Lafayette F-63000 Clermont-Ferrand (FR) @ Date of publication of application : 17.07.91 Bulletin 91/29 74) Representative : Hesketh, Alan, Dr. et al European Patent Department Merck & Co., @ Designated Contracting States : Inc. Tertings Park Eastwick Road CH DE FR GB IT LI NL Harlow Essex, CM20 2QR (GB) © Applicant : LABORATOIRES MERCK, SHARP & DOHME-CHIBRET 3, Avenue Hoche F-75008 Paris (FR) (S) Fluid ophthalmic composition based on lipid microparticles containing at least one active principle. (57) There is described a fluid ophthalmic composition which comprises a suspension in a fluid dispersant medium of lipid microparticles containing at least one active principle. The composition enables improved availability of the active principle to be obtained as a result of high intraocular levels. 00 <0 CO Q. UJ Jouve, 18, rue Saint-Denis, 75001 PARIS EP 0 437 368 A1 FLUID OPHTHALMIC COMPOSITION BASED ON LIPID MICROPARTICLES CONTAINING AT LEAST ONE ACTIVE PRINCIPLE The present invention relates to a fluid ophthalmic composition. Many ophthalmic compositions are currently available in liquid or solid form, but none of them is, in fact, completely satisfactory. In effect, liquid ophthalmic compositions, although easy to use, have some drawbacks ; in particular, it is 5 difficult to obtain a sustained or delayed action of the active principle which they contain. -

NEW ZEALAND DATA SHEET 1. PRODUCT NAME IOPIDINE® (Apraclonidine Hydrochloride) Eye Drops 0.5%
NEW ZEALAND DATA SHEET 1. PRODUCT NAME IOPIDINE® (apraclonidine hydrochloride) Eye Drops 0.5%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each mL of Iopidine Eye Drops 0.5% contains apraclonidine hydrochloride 5.75 mg, equivalent to apraclonidine base 5 mg. Excipient with known effect Benzalkonium chloride 0.1 mg per 1 mL as a preservative. For the full list of excipients, see section 6.1. 2. PHARMACEUTICAL FORM Eye drops, solution, sterile, isotonic. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications Iopidine Eye Drops 0.5% are indicated for short-term adjunctive therapy in patients on maximally tolerated medical therapy who require additional IOP reduction. Patients on maximally tolerated medical therapy who are treated with Iopidine Eye Drops 0.5% to delay surgery should have frequent follow up examinations and treatment should be discontinued if the intraocular pressure rises significantly. The addition of Iopidine Eye Drops 0.5% to patients already using two aqueous suppressing drugs (i.e. beta-blocker plus carbonic anhydrase inhibitor) as part of their maximally tolerated medical therapy may not provide additional benefit. This is because apraclonidine is an aqueous-suppressing drug and the addition of a third aqueous suppressant may not significantly reduce IOP. The IOP-lowering efficacy of Iopidine Eye Drops 0.5% diminishes over time in some patients. This loss of effect, or tachyphylaxis, appears to be an individual occurrence with a variable time of onset and should be closely monitored. The benefit for most patients is less than one month. 4.2. Dose and method of administration Dose One drop of Iopidine Eye Drops 0.5% should be instilled into the affected eye(s) three times per day. -

NEW CONCEPTS in GLAUCOMA CARE TREATMENT Proceedings of the Fifteenth Annual Meeting & of the Optometric Glaucoma Society
NEW CONCEPTS IN GLAUCOMA CARE TREATMENT Proceedings of the Fifteenth Annual Meeting & of the Optometric Glaucoma Society INSIDE: • Virtual Reality Uses in Glaucoma • Questions Glaucoma Patients Ask • Pathogenesis of Glaucoma • Glaucoma Progression • Real-Time Aqueous Humor Outfl ow Imaginging APRIL 2017 REVIEW OF OPTOMETRY/APRIL 2017 1 0217_OGS_ja_3.22.indd 1 3/24/17 3:36 PM ro0417ogs_vyzulta.indd 1 3/20/17 1:57 PM NEW CONCEPTS IN GLAUCOMA CARE TREATMENT TABLE OF CONTENTS INTRODUCTORY REMARKS The 15th Annual Scientifi c Meeting of the Optometric Glaucoma Society (OGS), held Nov. 15 and 16, 2016, in Anaheim, Calif., brought 3 together some of the country’s top luminaries INTRODUCTORY REMARKS in the areas of glaucoma diagnosis, treatment, Highlights From the Annual Scientifi c assessment, and management. These individu- Meeting als shared groundbreaking research and the BY MURRAY FINGERET, OD latest clinical knowledge about glaucoma—con- sidered to be the top global eye burden by the World Health Organization. 4 Kicking things off in the President’s Lecture, PRESIDENT’S LECTURE Felipe A. Medeiros, MD, PhD, highlighted potential clinical applications for More Than a Video Game: Virtual virtual reality devices. These devices, being tested in simulation laboratories, Reality and Its Uses in Glaucoma could one day assist clinicians in assessing patients at risk for glaucoma and BY FELIPE A. MEDEIROS, MD, PHD in danger of falls and motor vehicle accidents due to visual fi eld loss. Make no mistake: These cutting-edge tools are not your techie’s virtual reality. Dr. Medeiros, in a separate lecture about glaucoma progression, unveiled 6 an innovative metric developed by his research group to measure functional PATIENT CARE and structural vision loss in glaucoma patients. -

Hyperlinked Region IX SOP for MWLCEMS
2 Region IX 2 0 0 STANDARD OPERATING PROCEDURES/ 1 1 STANDING MEDICAL ORDERS 9 9 McHenry Western Lake County EMS System Healthcare delivery requires structure (people, equipment, education) and process (policies, protocols, procedures) that, when integrated, produce a system (programs, organizations, cultures) that leads to optimal outcomes (patient survival and safety, quality, satisfaction). An effective system of care comprises all of these elements—structure, process, system, and patient outcomes—in a framework of continuous quality improvement (AHA, 2015). These protocols have been developed and approved through a collaborative process involving the Advocate Lutheran General; Greater Elgin Area, McHenry Western Lake County, Northwest Community, Amita Saint Joseph Hospital, and Southern Fox Valley EMS Systems to reduce variation in practice and establish a Region-wide System of care. They shall be used: as the written practice guidelines/pathways of care approved by the EMS Medical Directors (EMS MDs) to be initiated by System EMS personnel for off-line medical control. as the standing medical orders to be used by Emergency Communications Registered Nurses (ECRNs) when providing on-line medical control (OLMC). in medium to large scale multiple patient incidents, given that the usual and customary forms of communication are contraindicated as specified in the Region IX disaster plan. System members are authorized to implement these orders to their scope of practice. OLMC communication shall be established without endangering the patient. Under no circumstances shall emergency prehospital care be delayed while attempting to establish contact with a hospital. In the event that communications cannot be established, EMS personnel shall continue to provide care to the degree authorized by their license, these protocols, drugs/equipment available, and their scope of practice granted by the EMS MD in that System. -

0Bcore Safety Profile
Core Safety Profile Active substance: Levobunolol Pharmaceutical form(s)/strength: Eye drops solution/ 0,1%; 0,25%; 0,5%; 0,5% UD P-RMS: CZ/H/PSUR/0006/001 Date of FAR: 26.05.2009 4.2 Posology and method of administration Adults (including the elderly) Country specific posology and method of administration to be included. Children /.../ is not recommended for use in children due to lack of safety and efficacy data. If required, /.../ may be used with other agents to lower intra-ocular pressure. The use of two topical beta-adrenergic blocking agents is not recommended (see section 4.4). Intraocular pressure should be measured approximately four weeks after starting treatment with /.../ as a return to normal ocular pressure can take a few weeks. As with any eye drops, to reduce possible systemic absorption, it is recommended that the lachrymal sac is compressed at the medial canthus (punctual occlusion) for one minute. This should be performed immediately following the instillation of each drop. Transfer from other beta-blocking treatment When another beta blocking agent is being used treatment must be discontinued after a full day of therapy. Start treatment with /.../ the next day with X drop of /.../ topically applied into the conjunctival sac in the affected eye(s). If /.../ is to replace a combination of anti-glaucoma products, only a single product should be removed at a time. Use in renal and hepatic impairment Levobunolol hydrochloride has not been studied in patients with hepatic or renal impairment. Therefore, caution should be used in treating such patients (see section 4.4). -

Efficacy and Safety of the Fixed Combinations of Tafluprost/Timolol
www.nature.com/scientificreports OPEN Efcacy and safety of the fxed combinations of tafuprost/timolol and latanoprost/carteolol Received: 4 February 2019 Masahiro Fuwa, Atsushi Shimazaki, Masafumi Mieda, Naoko Yamashita, Takahiro Akaishi, Accepted: 7 May 2019 Takazumi Taniguchi & Masatomo Kato Published: xx xx xxxx In this study, we made a comparative efcacy and safety assessment of two diferent fxed combinations of drugs, viz., tafuprost/timolol (TAF/TIM) and latanoprost/carteolol (LAT/CAR), by determining their efects on intraocular pressure (IOP) in ocular normotensive monkeys and examining their toxic efects on ocular surface using human corneal epithelial cells. TAF/TIM was found to be more efective in lowering IOP for a longer duration compared to LAT/CAR. We found that the diference in the intensity of IOP-lowering efect was because of the diferences in the strength of timolol compared with that of carteolol as a beta-adrenergic antagonist and strength of tafuprost compared with that of latanoprost as a prostaglandin analogue. In addition, TAF/TIM showed much less cytotoxic efects compared to LAT/CAR on the human corneal epithelial cells. Our fndings showed that TAF/TIM is better than LAT/CAR with regard to the IOP-lowering efect in monkeys and toxicity on ocular surface. Glaucoma is a neurodegenerative disease of the eyes characterised by selective retinal ganglion cell loss, fol- lowed by progressive defects in visual feld, resulting in the principal cause of irreversible blindness worldwide1–4. Elevated intraocular pressure (IOP) is an important contributor for the progression of glaucoma, for which the current treatment primarily involves IOP reduction1,5–8. -
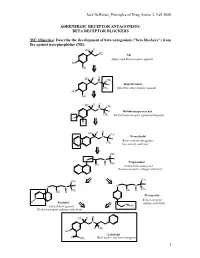
BETA RECEPTOR BLOCKERS MC Objective
Jack DeRuiter, Principles of Drug Action 2, Fall 2000 ADRENERGIC RECEPTOR ANTAGONISTS: BETA RECEPTOR BLOCKERS MC Objective: Describe the development of beta antagonists ("beta blockers") from the agonist norepinephrine (NE): HO H NH2 NE Alpha- and Beta-receptor agonist HO OH H H HO CH N 3 H Isoproterenol CH3 Selective beta-receptor agonist HO OH H H HO CH N 3 H Dichloroisoproterenol CH3 Partial beta-receptor agonist/antagonist Cl Cl H H HO CH N 3 Pronethalol H Beta-receptor antagonist, CH 3 low activity and toxic CH3 O N H Propranolol CH3 OH H Potent beta-antagonist No beta-receptor subtype selectivity CH3 CH3 O N H O N H CH CH OH H 3 OH H 3 Metoprolol N Beta-1-receptor H Pindolol O subtype selectivity Partial beta-agonist CH3 No beta-receptor subtype selectivity HO H H N H CH3 HO Labetolol O NH2 Dual alpha- and beta-antagonst 1 Jack DeRuiter, Principles of Drug Action 2, Fall 2000 MC Objective: Based on their structures, would the beta-blockers be expected to be relatively receptor selective? YES. They do not produce significant blockade of alpha- adrenergic receptors (alpha-1 or alpha-2), histamine receptors, muscarinic receptors or dopamine receptors. MC/PC Objective: Identify which beta blockers are classified as "non-selective": · The “non-selective" classification refers to those beta-blockers capable of blocking BOTH beta-1 and beta-2 receptors with equivalent efficacy. These drugs DO NOT have clinically significant affinity for other neurotransmitter receptors (alpha, dopamine, histamine, acetylcholine, etc.). · ALL of these beta-blockers (except satolol) consist of an aryloxypropanolamine side chain linked to an aromatic or “heteroaromatic” ring which is “ortho” substituted. -

Antiglaucoma Compositions Containing Combinations of Alpha-2 Agonists and Beta-Blockers
~" ' MM II II II MM II II II Ml II II I II J European Patent Office _ _ _ © Publication number: 0 365 662 B1 Office europeen* des.. brevets , © EUROPEAN PATENT SPECIFICATION © Date of publication of patent specification: 09.03.94 © Int. CI.5: A61 K 31/47 © Application number: 89905874.7 @ Date of filing: 26.04.89 © International application number: PCT/US89/01994 © International publication number: WO 89/10126 (02.11.89 89/26) (54) ANTIGLAUCOMA COMPOSITIONS CONTAINING COMBINATIONS OF ALPHA-2 AGONISTS AND BETA-BLOCKERS. ® Priority: 26.04.88 US 186504 1075-1078 @ Date of publication of application: J. of Cardiovascular Pharmacology, vol. 2, 02.05.90 Bulletin 90/18 suppl. 1, 1980, S. 21-28 © Publication of the grant of the patent: © Proprietor: ALCON LABORATORIES INC 09.03.94 Bulletin 94/10 6201 South Freeway Ft. Worth, TX 76134(US) © Designated Contracting States: AT BE CH DE FR GB IT LI LU NL SE @ Inventor: DE SANTIS, Louis, M. 2316 Wlnton Terrace West © References cited: Fort Worth, TX 761 09(US) US-A- 4 455 317 US-A- 4 515 800 © Representative: Jump, Timothy John Simon et AM A DRUG EVALUATION, 2nd ed., 1973; al "Agent used to treat Glaucoma", pp. 675-686. Venner Shipley & Co. 20 Little Britain GLAUCOMA, vol. 1, no. 1, February 1979; London EC1A 7DH (GB) 00 S.SUGAR, pp. 9-15. CM CO Ophthalmology, vol. 96, no. 1, 1989, p. 3-7 CO m Ophthalmology, vol. 98, no. 7, 1991, p. CO 00 Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole