Vibrio Cholerae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vincas Kudirka, Martynas Jankus, Jonas Šliūpas and the Making of Modern Lithuania Charles C
Georgia State University ScholarWorks @ Georgia State University History Dissertations Department of History Summer 2013 Lithuanians in the Shadow of Three Eagles: Vincas Kudirka, Martynas Jankus, Jonas Šliūpas and the Making of Modern Lithuania Charles C. Perrin Georgia State University Follow this and additional works at: https://scholarworks.gsu.edu/history_diss Recommended Citation Perrin, Charles C., "Lithuanians in the Shadow of Three Eagles: Vincas Kudirka, Martynas Jankus, Jonas Šliūpas and the Making of Modern Lithuania." Dissertation, Georgia State University, 2013. https://scholarworks.gsu.edu/history_diss/35 This Dissertation is brought to you for free and open access by the Department of History at ScholarWorks @ Georgia State University. It has been accepted for inclusion in History Dissertations by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. LITHUANIANS IN THE SHADOW OF THREE EAGLES: VINCAS KUDIRKA, MARTYNAS JANKUS, JONAS ŠLIŪPAS AND THE MAKING OF MODERN LITHUANIA by CHARLES PERRIN Under the Direction of Hugh Hudson ABSTRACT The Lithuanian national movement in the late nineteenth and early twentieth centuries was an international phenomenon involving Lithuanian communities in three countries: Russia, Germany and the United States. To capture the international dimension of the Lithuanian na- tional movement this study offers biographies of three activists in the movement, each of whom spent a significant amount of time living in one of -

Table of Contents
Table of Contents Schedule-at-a-Glance . 2 General Chairs’ Welcome Letter . 3 Conference Services . 5 Sponsoring Societies’ Booths . 7 Conference Materials . 8 Plenary Sessions and Awards Ceremony . 9 Special Symposia . 13 Applications & Technology Topical Reviews . 16 Short Courses . 19 Special Events . 29 CLEO:EXPO Exhibitors . 32 CLEO: Industry Focus . 34 CLEO Committees . 36 Explanation of Session/Presentation Codes . 41 Agenda of Sessions . 41 Technical Program Abstracts . 52 Key to Authors . 250 CLEO Management thanks the following corporate sponsors for their generous support: Conference on Lasers and Electro-Optics® CLEO • 13–18 May 2018 1 Schedule-at-a-Glance Sunday Monday Tuesday Wednesday Thursday Friday 13 May 14 May 15 May 16 May 17 May 18 May GENERAL Registration 07:00–17:00 07:00–18:00 07:00–18:30 07:30–18:30 07:30–18:00 07:30–15:30 Speaker Ready Room 13:00–17:00 07:00–18:00 07:00–18:00 07:00–17:30 07:00–18:00 07:30–15:30 Coffee Breaks 10:00–10:30 10:00–11:30 * 10:00–11:30* 10:00–11:30* 10:00–10:30 *on show floor 15:30–16:00 15:00–17:00* 15:00–17:00* 16:00–16:30 CLEO TECHNICAL PROGRAMMING Short Courses 08:30–17:30 12:30–16:30 12:00–16:00 Technical Sessions 08:00–18:00 13:00–19:00 13:00–19:00 08:00–18:30 08:00–16:00 Special Symposium and A&T 08:00–12:30 13:00–19:00 13:00–19:00 08:00–18:30 08:00–16:00 Topical Reviews Plenary Sessions 08:00–10:00 08:00–10:00 NEW Dynamic e-Posters 11:30–13:00 11:30–13:00 11:30–13:00 Postdeadline Paper Sessions 20:00–22:00 CLEO:EXPO AND SHOW FLOOR ACTIVITIES CLEO:EXPO 10:00–17:00 10:00–17:00 -

Ultrafast Fiber Lasers Enabled by Highly Nonlinear Pulse Evolutions
ULTRAFAST FIBER LASERS ENABLED BY HIGHLY NONLINEAR PULSE EVOLUTIONS A Dissertation Presented to the Faculty of the Graduate School of Cornell University in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy by Walter Pupin Fu August 2019 c 2019 Walter Pupin Fu ALL RIGHTS RESERVED ULTRAFAST FIBER LASERS ENABLED BY HIGHLY NONLINEAR PULSE EVOLUTIONS Walter Pupin Fu, Ph.D. Cornell University 2019 Ultrafast lasers have had tremendous impact on both science and applications, far beyond what their inventors could have imagined. Commercially-available solid-state lasers can readily generate coherent pulses lasting only a few tens of femtoseconds. The availability of such short pulses, and the huge peak in- tensities they enable, has allowed scientists and engineers to probe and manip- ulate materials to an unprecedented degree. Nevertheless, the scope of these advances has been curtailed by the complexity, size, and unreliability of such devices. For all the progress that laser science has made, most ultrafast lasers remain bulky, solid-state systems prone to misalignments during heavy use. The advent of fiber lasers with capabilities approaching that of traditional, solid-state lasers offers one means of solving these problems. Fiber systems can be fully integrated to be alignment-free, while their waveguide structure en- sures nearly perfect beam quality. However, these advantages come at a cost: the tight confinement and long interaction lengths make both linear and non- linear effects significant in shaping pulses. Much research over the past few decades has been devoted to harnessing and managing these effects in the pur- suit of fiber lasers with higher powers, stronger intensities, and shorter pulse durations. -

COLLEGE DEPT NAME/RANK MERIT AS Agricultural Sciences Marcy M
COLLEGE DEPT NAME/RANK MERIT AS Agricultural Sciences Marcy M. Beverly, Assistant Professor 500 AS Agricultural Sciences Roger D. Hanagriff, Assistant Professor 1,500 AS Agricultural Sciences William R. Harrell, Professor 2,000 AS Agricultural Sciences Thomas D. Higgins, Associate Professor/Coordinator 1,000 AS Agricultural Sciences Stanley F. Kelley, Associate Professor/Coordinator 1,500 AS Agricultural Sciences Robert A. Lane, Professor/Department Chair 2,500 AS Agricultural Sciences Billy Mac Moore, Professor 1,000 AS Agricultural Sciences Joe E. Muller, Associate Professor 1,000 AS Agricultural Sciences Nedom C. Muns, Professor 750 AS Agricultural Sciences Dwayne Pavelock, Assistant Professor 2,000 AS Agricultural Sciences Carolyn W. Robinson, Assistant Professor 1,000 AS Agricultural Sciences Douglas R. Ullrich, Jr., Associate Professor 1,250 AS Agricultural Sciences Barry L. Williams, Assistant Professor 0 AS Art Martin F. Amorous, II, Associate Professor 1,500 AS Art John D. Barnosky, Associate Professor 1,500 AS Art Mary K. Borcherding, Associate Professor 2,000 AS Art Charlotte M. Drumm, Assistant Professor 1,500 AS Art Michael H. Henderson, Assistant Professor 1,500 AS Art O. Emmette Jackson, Professor 1,000 AS Art Sharon A. King, Assistant Professor/Department Chair 3,000 AS Art Patric K. Lawler, Associate Professor 0 AS Art James E. Paster, Professor 0 AS Art Thomas A. Seifert, Associate Professor 1,500 AS Art Tony R. Shipp, Associate Professor 1,500 AS Biological Sciences Karolis R. Bagdonas, Associate Professor 0 AS Biological Sciences Theodore J. Brummel, Assistant Professor 2,500 AS Biological Sciences Jerry L. Cook, Assistant Professor 3,500 AS Biological Sciences Tamara J. -

Player No Surname First Name Assoc. 104 HOVHANNISYAN Melkon ARM
JUNIOR BOYS' PARTICIPANTS' LIST Player No Surname First name Assoc. 104 HOVHANNISYAN Melkon ARM 105 RAFAYELYAN Edgar ARM 110 BINDER Michael AUT 113 KOLODZIEJCZYK Maciej AUT 114 PROMBERGER Jonas AUT 115 ZILLER Thomas AUT 123 ANSARI Mahammad Ibrahim AZE 124 GAFARLI Yusif AZE 125 WANG Chenxi AZE 126 YANG Xinyu AZE 127 YU Khinhang AZE 133 COMELIAU David BEL 135 DEVOS Laurens BEL 136 GASPAR Romain BEL 137 JACQUES Quentin BEL 138 KOSOLOSKY Olav BEL 149 MIHAILOVIC Nikola BIH 150 MIHAILOVIĆ Luka BIH 161 KORTCHINSKI Ilia BLR 162 KUNATS Heorhi BLR 163 RUKLIATSOU Uladzislau BLR 173 PETROV Martin BUL 185 BANEK Mario CRO 187 JAKELIC Jakov CRO 188 KRSTEVSKI Aleks CRO 189 TICA Jakov CRO 200 CHRYSOSTOMOU Christos CYP 201 ELIA Iosif CYP 204 GEORGIOU Stylianos CYP 206 SAVVA Christos CYP 208 TSISSIOS Charalambos CYP 216 BAKO Radim CZE 219 MARTINKO Tomas CZE 220 ONDERKA Frantisek CZE 221 PRUSA David CZE 222 SKALA Radek CZE 232 ANDERSEN Martin DEN 1 233 CHRISTENSEN Thor DEN 234 SIMONSEN Daniel DEN 235 SVENNINGSEN Peter DEN 236 CLARK Joseph ENG 250 GUTIERREZ Marc ESP 251 LILLO Alberto ESP 253 RUIZ Francisco Miguel ESP 254 SORIA Javier ESP 264 LUUK Mart EST 266 STROGOV Stanislav EST 267 VUHKA Maksim EST 274 MORADABBASI Pedram FIN 275 NAUMI Alex FIN 276 PIHKALA Arttu FIN 281 BARDET Lilian FRA 282 BERTRAND Irvin FRA 283 DE NODREST Leo FRA 287 REMBERT Bastien FRA 288 ROLLAND Jules FRA 306 MEISSNER Cedric GER 307 MENG Fanbo GER 308 OEHME Benno GER 309 STUMPER Kay GER 310 WETZEL Felix GER 322 DAMIANIS Ioannis GRE 323 DIAMANTOPOULOS Michail GRE 325 SGOUROPOULOS Ioannis -

Polygenic Scores for Handedness and Their Association with Asymmetries in Brain Structure
Polygenic scores for handedness and their association with asymmetries in brain structure Sebastian Ocklenburg ( [email protected] ) Ruhr-Universitat Bochum https://orcid.org/0000-0001-5882-3200 Dorothea Metzen Ruhr University Bochum: Ruhr-Universitat Bochum Caroline Schlüter Ruhr University Bochum: Ruhr-Universitat Bochum Christoph Fraenz IfADo: Leibniz-Institut fur Arbeitsforschung an der TU Dortmund Larissa Arning Ruhr University Bochum: Ruhr-Universitat Bochum Fabian Streit Central Institute of Mental Health: Zentralinstitut fur Seelische Gesundheit Onur Güntürkün Ruhr University Bochum: Ruhr-Universitat Bochum Robert Kumsta Ruhr University Bochum: Ruhr-Universitat Bochum Erhan Genc IfADo: Leibniz-Institut fur Arbeitsforschung an der TU Dortmund Research Article Keywords: Handedness, laterality, hemispheric asymmetry, polygenic scores, genetics, motor Posted Date: March 23rd, 2021 DOI: https://doi.org/10.21203/rs.3.rs-350445/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at Brain Structure and Function on July 8th, 2021. See the published version at https://doi.org/10.1007/s00429-021-02335-3. Page 1/23 Abstract Handedness is the most widely investigated motor preference in humans. The genetics of handedness and especially the link between genetic variation, brain structure and right-left preference have not been investigated in detail. Recently, several well-powered genome-wide association studies (GWAS) on handedness have been published, signicantly advancing the understanding of the genetic determinants of left- and right-handedness. In the present study, we estimated polygenic scores (PGS) of handedness based on the latest GWAS by de Kovel and Francks (2019) in an independent validation cohort (n = 296). -

Undergraduate Commencement One Hundred and Fifty First Year 2014
La Salle University La Salle University Digital Commons La Salle Commencement Programs University Publications 2014 Undergraduate Commencement One Hundred and Fifty First Year 2014 La Salle University Follow this and additional works at: https://digitalcommons.lasalle.edu/commencement_programs LaSalle university ONE HUNDRED AND FIFTY-FIRST YEAR COMMENCEMENT Two T housand Fourteen r Undergraduate Commencement Exercises Sunday, May 18, 2014 William R. Sautter, Chairman, La Salle University Board o f Trustees, Presiding PROCESSIONAL (Pomp and Circumstance)* Ed w ard Elgar In v o ca tio n * ........................................... ..................................................................... Catalina Ta N ational An th em * ............................. ...........................................................FRANCIS SCOTT KEY Introduction of Stud ent Speaker ...................................................James E. Moore, Ph.D. Vice President for Student Affairs and Dean of Students A Graduate Sp e a k s ............................. ..........................................................Emily Rose Moran Conferring of h o n o r a ry Degree ..........................................................William R. Sautter Chair, Board of Trustees PRESENTATION OF LlNDBACK AWARD Joseph R. Marbach, Ph.D. Provost (The Christian R. and Mary F. Lindback Award is presented for Distinguished Teaching) PRESENTATION OF CANDIDATES ..............................................................................................................Joseph -
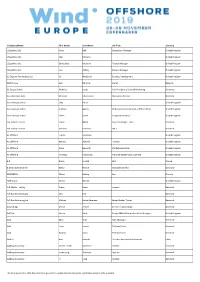
Company Name First Name Last Name Job Title Country
Company Name First Name Last Name Job Title Country 1StopWind Ltd Arran Bell Operations Manager United Kingdom 1StopWind Ltd. Alan Mckerns United Kingdom 1StopWind Ltd. Bernadette McAulay Finance Manager United Kingdom 1StopWind Ltd. Joel Telling General Manager United Kingdom 23 Degrees Renewables Ltd Ed Woodrow Business Development United Kingdom 24SEA bvba Gert De Sitter Owner Belgium 3S Europe GmbH Matthias Lamp Vice President of Sales & Marketing Germany 3sun Denmark ApS Christian Christensen Operations Director Denmark 3sun Group Limited Jody Potter United Kingdom 3sun Group Limited Graham Hacon VP Business Development, Offshore Wind United Kingdom 3sun Group Limited Sherri Smith Company Secretary United Kingdom 3W Industri Service Simon Øland Project manager - sales Denmark 3W Industri Service Kenneth Pedersen IWI-S Denmark 4C Offshore Lauren Anderson United Kingdom 4C Offshore Richard Aukland Director United Kingdom 4C Offshore Rosie Haworth Market Researcher United Kingdom 4C Offshore Vincenzo Poidomani Principal Geotechnical Engineer United Kingdom 8.2 Bruno ALLAIN CEO France 8.2 Monitoring GmbH Bernd Höring Managing director Germany 920338402 Ellinor Meling Ceo Norway A&P Group Emma Harrick United Kingdom A.P. Møller Holding Simon Ibsen Investor Denmark A/S Dan-Bunkering Ltd. Jens Kirk Denmark A/S Dan-Bunkering Ltd. Michael Brunø-Sørensen Senior Bunker Trader Denmark A1wind Aps Martin Jensen Director / A1wind Aps Denmark AAF Ltd Steven Brett Europe MFAS Aftermarket Sales Manager United Kingdom AAG Allan Tarp Sales Manager Denmark -
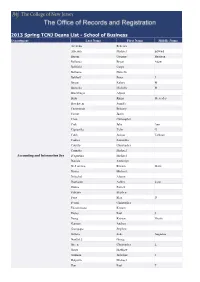
Cadenza Document
2013 Spring TCNJ Deans List - School of Business Department Last Name First Name Middle Name Acevedo Rebecca Alberque Michael Edward Baroni Gregory Harrison Bellanca Bryan Adam Bellfield Curyn Bellomo Danielle Belthoff Peter J Berger Kelsey M Bertscha Michelle M Blochlinger Alyssa Brito Rhina Mercedes Bruckstein Jennifer Cammarota Brittany Cantor Justin Chan Christopher Ciak Julie Ann Cignarella Tyler G Cobb Joshua Tallman Codner Samantha Cotrufo Christopher Cozzetta Michael Accounting and Information Sys D'agostino Michael Daniels Amberlyn De Lorenzo Kristen Marie Divito Michael Dritschel Alyson Dunhamn Ashley Lynn Dunne Patrick Fabiano Stephen Faris Elen D Ferrari Christopher Fitzsimmons Kristen Flores Paul S Foerg Kristen Nicole Gannon Andrew Giampapa Stephen Gillette Zeke Augustus Gonzalez George Green Christopher L Gross Matthew Guzman Jackeline J Halperin Michael Han Paul T Department Last Name First Name Middle Name Heaney Tara L Heindel Robert Henderson Molly Herlihy Bryan Hernberg Matthew Hicks Melody Nicole Holroyd Faith Anne Hosterman Tyler Jennings Amy Elizabeth Jordan Brian Patrick Kettelkamp Kara Ann Kleczynski Joseph Klein Brandon Kremenich Brian Edward Leming Steven Lis Amy Lok Kathleen Marcus Erik Joseph Matulis Karolis McConaghie Cara Mehaffey Michael Charles Mikulicz Taryn Murray John Ng Ryan Accounting and Information Sys O'Dowd Kevin Olivola Michael B Oriolo Greg Pacione Alexander Palantone Michael V Patel Aesha Pica Joseph Picciano Deanna Pokusa Shannon Christine Pousatis Alannah K Previti Helene Redes Kayla Marie -

Student Name Grade Ackerman, Lucas J. 6 Anderson, Ethan G
STUDENT NAME GRADE ACKERMAN, LUCAS J. 6 ANDERSON, ETHAN G. 6 ANDERSON, MAXIM J. 6 ANIMASAUN, OLUWATOMI S. 6 ASRAT, LIDIA T. 6 ATCHISON, HALEY L. 6 AUTREY, CAMDEN T. 6 BACHA, LINCOLN D. 6 BAKKE, IRENE K. 6 BAKKE, JULIA L. 6 BALTZER, JACOB T. 6 BARKER, NATHANIEL X. 6 BARTSCH, SYDNEY E. 6 BECK, OLIVER E. 6 BELL, GIANNA R. 6 BELZ, LAURYN L. 6 BENGTSON, SKYLER D. 6 BENZ, MAGNUS R. 6 BERENDT, CHRISTIAN R. 6 BERTELSEN, ZACHARY W. 6 BLAUER, CLARA M. 6 BRADSHAW, MORGAN L. 6 BUCHE, JAKE H. 6 BUCKLEY, AVERY K. 6 BURBACK, KAYA M. 6 CANNON, CHARLOTTE F. 6 CANTWELL, ALEXANDER T. 6 CAPUTO, CHARLOTTE E. 6 CASTRO, MADELYN E. 6 CELSKI, NATHANIEL M. 6 CENEDESE, GIORGIA LUZIA 6 CESSNA, CARTER D. 6 CHERRIER, EVAN P. 6 CHRISTENSEN, ETHAN C. 6 CLARK, CAITLYN S. 6 CLARK, RIO D. 6 COOPET, TEAGAN C. 6 CURREN, OLIVER J. 6 DARGAY, ELLA R. 6 DAVIS, SEANNA R. 6 DEBAUCHE, HAZEL L. 6 DEMENY, KILEY M. 6 DERMODY, CYRUS F. 6 DEWITT, LILY M. 6 DUFRESNE, ANDREW C. 6 DUFRESNE, HAZEL M. 6 DUSING, CLAIRE N. 6 EDER, SAMANTHA R. 6 FATIMA, SHAZIA 6 FAWCETT, HENRY W. 6 FITZSIMMONS, KEIRA J. 6 FLIEHLER, LANEY 6 FLOOD, HOLLY E. 6 FROST, RYLEE E. 6 GAJESKI, GABBY J. 6 GALE, CHLOE K. 6 GALLAGHER, AUDREY L. 6 GERDING, GRAYSON J. 6 GIBSON, LOGAN L. 6 GLASOW, ABIGAIL R. 6 GRAWE, OLIVER G. 6 GRENDAHL, AIDAN M. 6 GRIEFENHAGEN, SOPHIA R. 6 GRIFFIN, CARLIE J. 6 GRIFFIN, KEENAN P. -

COLLEGE DEPT NAME/RANK MARK A&S Agricultural and Industrial Sciences Marcy M. Beverly, Assistant Professor 1,000 A&S
COLLEGE DEPT NAME/RANK MARK A&S Agricultural and Industrial Sciences Marcy M. Beverly, Assistant Professor 1,000 A&S Agricultural and Industrial Sciences Roger D. Hanagriff, Associate Professor A&S Agricultural and Industrial Sciences Stanley F. Kelley, Associate Professor/Coordinator A&S Agricultural and Industrial Sciences Douglas M. Kingman, Assistant Professor A&S Agricultural and Industrial Sciences Robert A. Lane, Professor A&S Agricultural and Industrial Sciences Michael H. Lau, Assistant Professor A&S Agricultural and Industrial Sciences Matthew L. McMillan, Assistant Professor A&S Agricultural and Industrial Sciences Joe E. Muller, Associate Professor A&S Agricultural and Industrial Sciences Nedom C. Muns, Professor A&S Agricultural and Industrial Sciences Dwayne Pavelock, Assistant Professor 1,000 A&S Agricultural and Industrial Sciences Douglas R. Ullrich, Jr., Associate Professor/Acting Chair A&S Art Martin F. Amorous, II, Associate Professor A&S Art John D. Barnosky, Associate Professor A&S Art Brian J. Benfer, Assistant Professor A&S Art Mary K. Borcherding, Professor A&S Art David S. Dawson, Assistant Professor A&S Art Charlotte M. Drumm, Associate Professor A&S Art Rebecca L. Finley, Assistant Professor A&S Art Matthew A. Guest, Assistant Professor A&S Art Michael H. Henderson, Assistant Professor A&S Art O. Emmette Jackson, Professor A&S Art Taehee Kim, Assistant Professor A&S Art Sharon A. King, Assistant Professor A&S Art Patric K. Lawler, Associate Professor A&S Art James E. Paster, Professor A&S Art Thomas A. Seifert, Associate Professor A&S Art Tony R. Shipp, Associate Professor/Acting Chair 5,000 A&S Biological Sciences Karolis R. -

Exam Rate Name Command Short Title ABE1 AMETO YAOVI AZO
Exam Rate Name Command Short Title ABE1 AMETO YAOVI AZO USS JOHN C STENNIS ABE1 FATTY MUTARR TRANSITPERSU PUGET SOUND WA ABE1 GONZALES BRIAN USS NIMITZ ABE1 GRANTHAM MASON USS DWIGHT D EISENHOWER ABE1 HO TRAN HUYNH B TRANSITPERSU PUGET SOUND WA ABE1 IVIE CASEY TERR NAS JACKSONVILLE FL ABE1 LAXAMANA KAMYLL USS GERALD R FORD CVN-78 ABE1 MORENO ALBERTO NAVCRUITDIST CHICAGO IL ABE1 ONEAL CHAMONE C PERSUPP DET NORTH ISLAND CA ABE1 PINTORE JOHN MA USS GEORGE H W BUSH ABE1 RIVERA MARIANI USS THEODORE ROOSEVELT ABE1 ROMERO ESPERANZ NOSC SAN DIEGO CA ABE1 SANMIGUEL MICHA USS GEORGE H W BUSH ABE1 SANTOS ANGELA V USS CARL VINSON ABE2 ANTOINE BRODRIC PERSUPPDET KEY WEST FL ABE2 AUSTIN ARMANI V USS RONALD REAGAN ABE2 AYOUB FADI ZEYA USS CARL VINSON ABE2 BAKER KATHLEEN USS ABRAHAM LINCOLN ABE2 BARNABE ALEXAND USS RONALD REAGAN ABE2 BEATON TOWAANA USS ABRAHAM LINCOLN ABE2 BEDOYA NICOLE USS THEODORE ROOSEVELT ABE2 BIRDPEREZ ZULYR HELICOPTER MINE COUNT SQ 12 VA ABE2 BLANCO FERNANDO USS GEORGE WASHINGTON ABE2 BRAMWELL ALEXAR USS HARRY S TRUMAN ABE2 CARBY TAVOY KAM PERSUPPDET KEY WEST FL ABE2 CARRANZA KEKOAK USS GEORGE WASHINGTON ABE2 CASTRO BENJAMIN USS THEODORE ROOSEVELT ABE2 CIPRIANO IRICE USS NIMITZ ABE2 CONNER MATTHEW USS JOHN C STENNIS ABE2 DOVE JESSICA PA USS THEODORE ROOSEVELT ABE2 DREXLER WILLIAM PERSUPP DET CHINA LAKE CA ABE2 DUDREY SARAH JO USS GEORGE H W BUSH ABE2 FERNANDEZ ROBER USS THEODORE ROOSEVELT ABE2 GAL DANIEL USS GEORGE H W BUSH ABE2 GARCIA ALEXANDE NAS LEMOORE CA ABE2 GREENE DONOVAN USS RONALD REAGAN ABE2 HALL CASSIDY RA USS THEODORE