Influence of Androgens on Circulating Adiponectin in Male and Female Rodents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MMC International BV
M.M.C. International Steroid Substances Steroid Test A Colour Steroid Test B Colour Steroid Test B Colour with UV Light Stanozolol/ Oxandrolone Test Clenbuterol/ Oxymetholone Test Ephedrine Test Alfadolone Orange Yellow Nil - - - Androsterone Orange Yellow White - - - Beclometasone Brown–yellow Orange Nil - - - Betamethasone Orange–brown Pink–Orange Nil - - - Boldenone Base (Equipoise, Ganabol) (pure powder) Warm red after 2 min. Dark Orange after 2 min. Bright Light Orange - - - Boldenone Undecanoate (oil) Dark brownish-red Dark Red Bright Light Orange - - - Boldenone Undecylenate (oil) Orange - Light Brown Dark Orange → Brown Bright Light Orange-Yellow - - - Carbenoxolone (CBX) Orange Yellow Yellow - - - Cholesterol Violet Orange White - - - Clenbuterol (Spiropent, Ventipulmin) - - - - Purple - Dark brown with yellow-green on the Dark brown with yellow-green on the Clomiphene (Androxal, Clomid, Omifin) Nil Dark brown to black No reaction Dark brown to black sides of the ampoule sides of the ampoule Cortisone Orange Yellow Green - - - Desoxycortone Blue–black Yellow Yellow - - - Dexamethasone Yellow Orange–pink Nil - - - Dienestrol Yellow Orange–red Nil - - - Diethylstilbestrol (DES) Orange (→yellow–green) Nil - - - Dimethisterone Brown–green Orange–red Yellow - - - Drostanolone Propionate (Masteron) (oil) Bright green Yellow-Orange Orange - - - Dydrogesterone (Duphaston) - Orange Green-Yellow - - - Enoxolone Orange Yellow Green-Yellow - - - Ephedrine (also for Pseudo- and Nor-Ephedrine) - - - - - Orange Estradiol (Oestradiol) Orange -

Toiminta Unita on Ulla La Mungukurti |
TOIMINTAUNITA USON 20180071390A1ULLA LA MUNGUKURTI | ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2018/ 0071390 A1 PATEL et al. (43 ) Pub . Date : Mar . 15 , 2018 ( 54 ) COMPOSITIONS OF PHARMACEUTICAL A61K 9 / 06 (2006 .01 ) ACTIVES CONTAINING DIETHYLENE A61K 9 /00 (2006 .01 ) GLYCOL MONOETHYL ETHER OR OTHER A61K 31 /573 ( 2006 .01 ) ALKYL DERIVATIVES A61K 31/ 565 ( 2006 .01 ) A61K 31/ 4439 ( 2006 . 01 ) ( 71 ) Applicant : THEMIS MEDICARE LIMITED , A61K 31 / 167 ( 2006 . 01 ) Mumbai (IN ) A61K 31 / 57 (2006 . 01) (52 ) U . S . CI. (72 ) Inventors : Dinesh Shantilal PATEL , Mumbai CPC .. .. .. A61K 47 / 10 ( 2013 . 01 ) ; A61K 9 /4858 ( IN ) ; Sachin Dinesh PATEL , Mumbai ( 2013 .01 ) ; A61K 9 /08 ( 2013 .01 ) ; A61K 9 / 06 ( IN ) ; Shashikant Prabhudas ( 2013 .01 ) ; A61K 9 / 0014 ( 2013 .01 ) ; A61K KURANI, Mumbai ( IN ) ; Madhavlal 31/ 573 ( 2013 .01 ) ; A61K 31 /57 ( 2013 .01 ) ; Govindlal PATEL , Mumbai ( IN ) A61K 31/ 565 ( 2013 .01 ) ; A61K 31 /4439 (73 ) Assignee : THEMIS MEDICARE LIMITED , ( 2013 .01 ) ; A61K 31/ 167 ( 2013 .01 ) ; A61K Mumbai (IN ) 9 /0048 ( 2013 .01 ) ; A61K 9 /0019 (2013 .01 ) ( 57 ) ABSTRACT (21 ) Appl. No .: 15 / 801, 390 The present invention relates to pharmaceutical composi tions of various pharmaceutical actives, especially lyophilic ( 22 ) Filed : Nov . 2 , 2017 and hydrophilic actives containing Diethylene glycol mono ethyl ether or other alkyl derivatives thereof as a primary Related U . S . Application Data vehicle and /or to pharmaceutical compositions utilizing (62 ) Division of application No. 14 /242 , 973 , filed on Apr. Diethylene glycol monoethyl ether or other alkyl derivatives 2 , 2014 , now Pat. No. 9 , 827 ,315 . -

(12) Patent Application Publication (10) Pub. No.: US 2014/0296.191 A1 PATEL Et Al
US 20140296.191A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0296.191 A1 PATEL et al. (43) Pub. Date: Oct. 2, 2014 (54) COMPOSITIONS OF PHARMACEUTICAL (52) U.S. Cl. ACTIVES CONTAINING DETHYLENE CPC ............... A61K 47/10 (2013.01); A61 K9/0019 GLYCOL MONOETHYLETHER OR OTHER (2013.01); A61 K9/0048 (2013.01); A61 K ALKYL DERVATIVES 45/06 (2013.01) USPC ........... 514/167: 514/177; 514/178: 514/450; (71) Applicant: THEMIS MEDICARE LIMITED, 514/334: 514/226.5: 514/449; 514/338; Mumbai (IN) 514/256; 514/570; 514/179; 514/174: 514/533; (72) Inventors: Dinesh Shantilal PATEL, Mumbai (IN); 514/629; 514/619 Sachin Dinesh PATEL, Mumbai (IN); Shashikant Prabhudas KURANI, Mumbai (IN); Madhavlal Govindlal (57) ABSTRACT PATEL, Mumbai (IN) (73) Assignee: THEMIS MEDICARE LIMITED, The present invention relates to pharmaceutical compositions Mumbai (IN) of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl (21) Appl. No.: 14/242,973 ether or other alkyl derivatives thereofas a primary vehicle and/or to pharmaceutical compositions utilizing Diethylene (22) Filed: Apr. 2, 2014 glycol monoethyl ether or other alkyl derivatives thereofas a primary vehicle or as a solvent system in preparation of Such (30) Foreign Application Priority Data pharmaceutical compositions. The pharmaceutical composi Apr. 2, 2013 (IN) ......................... 1287/MUMA2013 tions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formu Publication Classification lations containing such pharmaceutical actives and are Suit able for use as injectables for intravenous and intramuscular (51) Int. Cl. administration, as well as for use as a preformed solution/ A647/ (2006.01) liquid for filling in and preparation of capsules, tablets, nasal A6 IK 45/06 (2006.01) sprays, gargles, dermal applications, gels, topicals, liquid oral A6 IK9/00 (2006.01) dosage forms and other dosage forms. -

SEAC) Held on 01/10/2019 at Committee Room, Gujarat Pollution Control Board, Sector 10A,Gandhinagar
Minutes of the 554 th meeting ofthe State Level Expert Appraisal Committee (SEAC) held on 01/10/2019 at Committee Room, Gujarat Pollution Control Board, Sector 10A,Gandhinagar. The 554 th meeting of the State Level Expert Appraisal Committee (SEAC) was held on 1st October 2019 at Committee Room, Gujarat Pollution Control Board, Sector 10A, Gandhinagar. Following members attended the meeting: 1. Dr. Dinesh Misra, Chairman, SEAC 2. Shri S. C. Srivastav, Vice Chairman, SEAC 3. Shri Rajesh. I. Shah, Member, SEAC 4. Shri V. N. Patel, Member, SEAC 5. Dr. V. K. Jain, Member, SEAC 6. Shri A. K. Mule, Member, SEAC The regular agenda of Appraisal, Screening & Scoping/ToR, Reconsideration cases were taken up. The Committee considered the applications made by project proponents, additional details submitted as required by the SEAC/SEIAA and details furnished in the Form-1, PFR, EIA-EMP reports. Proposal No. Name and Address of the Unit Remarks 04 SIA/GJ/IND2/29059/2017 M/s. Dev Dyechem Industries EC – Plot No. C-1/324, Phase-I & II GIDC – Reconsideration Naroda, Ahmedabad. • PP remained absent in the EC – Appraisal presentation held on 01/10/2019. • After detailed discussion, Committee decided to defer the proposal and consider the same in one of the upcoming meeting of SEAC. 05 SIA/GJ/IND2/29678/2018 M/s. Apex Pharma EC – C-298/2/4, Saykha Industrial Estate, Reconsideration Saykha, Ta- Vagra, Dist -Bharuch. Category of the unit: 5(f) Project status: New • Project proponent (PP) submitted online application vide no. SIA/GJ/IND2/29678/2018 dated 30/04/19 for obtaining Environmental Clearance. -

NEWS 02 2020 ENG.Qxp Layout 1
Polymers and fluorescence Balance of power 360° drinking water analysis trilogy Fluorescence spectroscopy LCMS-8060NX: performance of industrial base polymers and robustness without Automatic, simultaneous and compromising sensitivity rapid analysis of pesticides and speed CONTENT APPLICATION »Plug und Play« disease screening solution? – The MALDI-8020 in screening for Sickle Cell Disease 4 Customized software solutions for any measurement – Macro programming for Shimadzu UV-Vis and FTIR 8 Ensuring steroid-free food supplements – Identification of steroids in pharmaceuticals and food supplements with LCMS-8045 11 MSn analysis of nonderivatized and Mtpp-derivatized peptides – Two recent studies applying LCMS-IT-TOF instruments 18 Polymers and fluorescence – Part 2: How much fluorescence does a polymer show during quality control? 26 PRODUCTS The balance of power – LCMS-8060NX balances enhanced performance and robustness 7 360° drinking water analysis: Episode 2 – Automatic, simul- taneous and rapid analysis of pesticides in drinking water by online SPE and UHPLC-MS/MS 14 Versatile testing tool for the automotive industry – Enrico Davoli with the PESI-MS system (research-use only [RUO] instrument) New HMV-G3 Series 17 No more headaches! A guide to choosing the perfect C18 column 22 Validated method for monoclonal antibody drugs – Assessment of the nSMOL methodology in Global solution through the validation of bevacizumab in human serum 24 global collaboration LATEST NEWS Global solution through global collaboration – Shimadzu Cancer diagnosis: -
EXECUTIVE SUMMARY Steroids
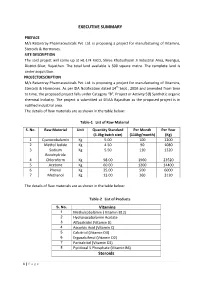
EXECUTIVE SUMMARY PREFACE M/s Ratantray Pharmaceuticals Pvt. Ltd. is proposing a project for manufacturing of Vitamins, Steroids & Hormones. SITE DESCRIPTION The said project will come up at H1-174 RIICO, Shree Khatushyam Ji Industrial Area, Reengus, District-Sikar, Rajasthan. The total land available is 500 square metre. The complete land is under acquisition. PROJECTDESCRIPTION M/s Ratantray Pharmaceuticals Pvt. Ltd. is proposing a project for manufacturing of Vitamins, Steroids & Hormones. As per EIA Notification dated 14th Sept., 2006 and amended from time to time, the proposed project falls under Category “B”, Project or Activity 5(f) Synthetic organic chemical Industry. The project is submitted at SEIAA Rajasthan as the proposed project is in notified industrial area. The details of Raw materials are as shown in the table below: Table-1 List of Raw Material S. No. Raw Material Unit Quantity Standard Per Month Per Year (5.0kg batch size) (110kg/month) (Kg) 1 Cyanocobalamin Kg 5.00 100 1200 2 Methyl Iodide Kg 4.50 90 1080 3 Sodium Kg 5.50 110 1320 Borohydride 4 Chloroform Kg 98.00 1960 23520 5 Acetone Kg 60.00 1200 14400 6 Phenol Kg 25.00 500 6000 7 Methanol Kg 13.00 260 3120 The details of Raw materials are as shown in the table below: Table-2 List of Products S. No. Vitamins 1 Methylcobalamin ( Vitamin B12) 2 Hydroxocobalamin Acetate 3 Alfacalcidol (Vitamin D) 4 Ascorbic Acid (Vitamin C) 5 Calcitriol (Vitamin D3) 6 Ergocalciferol (Vitamin D2) 7 Paricalcitol (Vitamin D2) 8 Pyridoxal 5 Phosphate (Vitamin B6) Steroids 1 | Page 1 Beclomethasone -

Injectable Steroids Oral Steroids Post Cycle Therapy Fat Burners Manufacturers Contact Us
Blog Shopping Cart Wishlist My Account Checkout Search $0.00 Menu STEROIDS SHOP INJECTABLE STEROIDS ORAL STEROIDS POST CYCLE THERAPY FAT BURNERS MANUFACTURERS CONTACT US Injectable Steroids Shop Injectable Steroids SORT BY POPULARITY Search SOLD OUT INJECTABLE STEROIDS Deca Durabolin Equipoise HGH Masteron NPP INJECTABLE STEROIDS Parabolan Androxine $90.00 Primobolan Depot Sustanon 250 Testosterone Cypionate Testosterone Enanthate Testosterone Propionate Testosterone Suspension Trenbolone Acetate Trenbolone Enanthate Trenbolone Suspension Tri Tren Winstrol Depot ORAL STEROIDS INJECTABLE STEROIDS Anadrol Aquaviron Anavar $66.00 Dianabol Halotestin Methyltrienolone Moda됍nil Primobolan Proviron Superdrol Testosterone Undecanoate Turinabol Winstrol POST CYCLE THERAPY Anastrozole EQUIPOISE Boldebolin Cabergoline $60.00 Clomid Exemestane HCG HMG Letrozole Nolvadex FAT BURNERS Clenbuterol Cytomel T3 MANUFACTURERS EQUIPOISE Alpha Pharma Boldebolin (vial) Eminence Labs $55.00 Maxtreme Pharma SOLD OUT EQUIPOISE BoldePrime $69.00 EQUIPOISE Bold-Max $73.00 SOLD OUT EQUIPOISE Bold-One $39.00 SOLD OUT INJECTABLE STEROIDS CypoPrime $43.00 SOLD OUT DECA DURABOLIN DecaPrime $54.00 INJECTABLE STEROIDS Alphabolin $62.00 INJECTABLE STEROIDS Alphabolin (vial) $125.00 SOLD OUT INJECTABLE STEROIDS EnaPrime $43.00 HGH Eutropin 4 IU $55.00 HGH Humatrope $550.00 INJECTABLE STEROIDS Induject-250 $59.00 INJECTABLE STEROIDS Induject-250 (vial) $59.00 SOLD OUT INJECTABLE STEROIDS DrostoPrime $72.00 INJECTABLE STEROIDS -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2020 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, following to be employed in commercial fishing or the commercial UST, MXT, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

Trenbolone Acetate”
STEROIDS ANTI SHOP INJECTABLE ORAL PCT HORMONES ESTROGENS ONLINE GENERIC BLOG Home / Products tagged “Trenbolone acetate” Search products… Trenbolone acetate SEARCH Dosage The rate of administration of the drug varies from 50 to 100 mg per day. However, the latter value is considered overpriced. Acetate in an amount of STEROIDS FOR 50 ml already gives a sufficient level of endurance and an increase in SALE muscle mass. If you do not have daily injections, you can limit yourself to taking 100 ml once every 2 days. In this case, the drug is not completely broken down by the liver. The use of a stimulant in small doses at short intervals makes it possible to increase its bioavailability. Side effects Among the side effects noted: pressure surges, the appearance of aggression and agitation, acne, cases of baldness and increased oily skin were recorded. When taken, the production of the body’s own hormone Alpha Pharma testosterone is suppressed, which leads to a decrease in libido and testicular atrophy. The steroid does not adversely affect the functioning of the Dragon Pharma kidneys, however, the color of the urine may turn red. The effect on the liver is considered moderate. Maxtreme Reception effects Indian Brand Regularly taking injections can achieve: BM Pharmaceuticals • A significant increase in muscle mass (up to 10 kg per course). • Increase the level of IGF by 200%. Healing Pharma • Improving power performance. • Intensive fat burning. Natco Pharma • Decreased cortisol levels. Sun Pharmaceuticals Showing all 6 Sort by popularity -

Annexure I: Legal Undertaking for Three Conditions
A-1 A-2 A-3 A-4 A-5 A-6 A-7 A-8 A-9 A-10 A-11 A-12 A-13 A-14 A-15 A-16 A-17 A-18 A-19 A-20 A-21 A-22 A-23 A-24 A-25 A-26 NAME OF PRODUCT : TESTOSTERONE MAXIMUM QUANTITY TO BE : 2.2605 MT/Month PRODUCED Raw Materials Require: Raw Materials Quantity in MT/Month (MAXIMUM) 3-Enol ether OR 4-AD 3- 2.9387 ethoxyandrosta-3,5-dien-17-one Ethanol 8.8160 Triethylamine 0.0723 Sodium Borohydride 0.6782 Hydrochloric acid 2.2605 Water 6.7815 Ethyl Acetate 6.7815 MDC 1.1303 Charcoal + Hyflow 0.0452 Brief Process: 3-Enol etherOR 4-AD 3-ethoxyandrosta-3,5-dien-17-one will be converted into 3-Enol hydroxyOR 4-AD 3-ethoxyandrosta-3,5-dien-17-ol in presence of sodium borohydride and triethylamine, further this compound converts into crude testosterone by treating with 10% hydrochloric acid. This crude material purified by charcoal treatment with organic solvents. CHEMICAL REACTIONS: A-27 1. TESTOSTERONE 4- AD -1.3 KG ETHANOL – 3.9 KG WATER 3.0 KG SODIUM BOROHYDRIDE- 0.3KG TRIETHYL AMINE - 0.032 KG HCL (10%) 1.0 KG 9.532 KG CRUDE TESTOSTERON eeTESTOSTERON FILTERATION ML-8.32 kg STRIPPER 1.2 KG 4.5kg Solvent ETHYL ACETATE – 3.0kg (Ethanol)-3.8kg KG MDC – 0.5 KG 4.72 KG CHARCOAL + HYFLOW – 0.02KG ETP / MEE FILTERATION Charcoal +Hyflow-0.02kg Solvent-3.44kg Residue-0.26kg Product – 1.0 kg A-28 NAME OF PRODUCT : TESTOSTERONE PROPIONATE MAXIMUM QUANTITY TO BE : 2.2605 MT/Month PRODUCED Raw Materials Require: Raw Materials Quantity in MT/Month (MAXIMUM) Testosterone 2.2605 MDC 22.605 Pyridine 2.2668 Propionyl chloride 0.9042 Methanol 2.2605 Brief Process: Testosterone propionate reacts with propionyl chloride and yields testosterone propionate in presence of organic solvent MDC and methylene chloride and pyridine which purified by chacoalization process in organic solvent methanol. -

Anabolic Steroids and Peptides Shop in USA
Anabolic Steroids and Peptides Shop in USA Steroids for Sale Shop Sexual Health Anti Estrogens Skin Weight Loss Hormones & Peptides Hair Loss PCT Gel About us Blog Checkout Cart Home / Products tagged “HCG” HCG Showing all 7 results Default sorting High Quality Gona-Max in USA $60.00 High Quality Fertigyn (Pregnyl) in USA High Quality Fertigyn HP 5000 in USA Read more $31.00 $39.00 Add to cart Add to cart High Quality HCG 10000IU in USA High Quality HCG 2000IU in USA High Quality HCG 5000IU in USA $39.00 $16.00 $24.00 Add to cart Add to cart Add to cart High Quality Ovidac 5000 IU in USA $39.00 Add to cart Search products… SEARCH Filter by price Filter Price: $10 — $60 Product categories Anti Estrogens Gel Hair Loss Hormones & Peptides Injectable Steroids Oral Steroids PCT Sexual Health Skin Uncategorized Weight Loss Product tags Acyclovir (Zovirax) Anastrozole Augmentin Azithromycin Boldenone undecylenate (Equipose) Cabergoline (Cabaser) Clenbuterol hydrochloride (Clen) Clomiphene citrate (Clomid) Drostanolone propionate (Masteron) Exemestane (Aromasin) Fluoxymesterone (Halotestin) Furosemide (Lasix) HCG Human Growth Hormone (HGH) Isotretinoin (Accutane) Letrozole Liothyronine (T3) Mesterolone (Proviron) Methandienone oral (Dianabol) Methenolone acetate (Primobolan) Methenolone enanthate (Primobolan depot) Methyl drostanolone (Superdrol) Methyltrienolone (Methyl trenbolone) Moda됍nil Nandrolone decanoate (Deca) Nandrolone phenylpropionate (NPP) Oxandrolone (Anavar) Oxymetholone (Anadrol) Sildena됍l Citrate Stanozolol injection (Winstrol depot) Stanozolol oral (Winstrol) Sustanon 250 (Testosterone mix) Tadala됍l Tamoxifen citrate (Nolvadex) Testosterone cypionate Testosterone enanthate Testosterone propionate Testosterone suspension Testosterone undecanoate Trenbolone acetate Trenbolone enanthate Trenbolone Enanthate, Testosterone Enanthate, Drostanolone Enanthate Trenbolone hexahydrobenzylcarbonate Trenbolone Mix (Tri Tren) Turinabol (4-Chlorodehydromethyltestosterone) Dentist WordPress Theme By VWThemes. -

Pharmaceutical and Veterinary Compounds and Metabolites
PHARMACEUTICAL AND VETERINARY COMPOUNDS AND METABOLITES High quality reference materials for analytical testing of pharmaceutical and veterinary compounds and metabolites. lgcstandards.com/drehrenstorfer [email protected] LGC Quality | ISO 17034 | ISO/IEC 17025 | ISO 9001 PHARMACEUTICAL AND VETERINARY COMPOUNDS AND METABOLITES What you need to know Pharmaceutical and veterinary medicines are essential for To facilitate the fair trade of food, and to ensure a consistent human and animal welfare, but their use can leave residues and evidence-based approach to consumer protection across in both the food chain and the environment. In a 2019 survey the globe, the Codex Alimentarius Commission (“Codex”) was of EU member states, the European Food Safety Authority established in 1963. Codex is a joint agency of the FAO (Food (EFSA) found that the number one food safety concern was and Agriculture Office of the United Nations) and the WHO the misuse of antibiotics, hormones and steroids in farm (World Health Organisation). It is responsible for producing animals. This is, in part, related to the issue of growing antibiotic and maintaining the Codex Alimentarius: a compendium of resistance in humans as a result of their potential overuse in standards, guidelines and codes of practice relating to food animals. This level of concern and increasing awareness of safety. The legal framework for the authorisation, distribution the risks associated with veterinary residues entering the food and control of Veterinary Medicinal Products (VMPs) varies chain has led to many regulatory bodies increasing surveillance from country to country, but certain common principles activities for pharmaceutical and veterinary residues in food and apply which are described in the Codex guidelines.