Issue 2 Jan-Mar 2014
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rosscarrde2017.Pdf (4.959Mb)
THE UNIVERSITY OF CENTRAL OKLAHOMA Edmond, OK Jackson College of Graduate Studies Method Development and Validation for Drug Identification and Confirmation by LC/MS-MS for Limited-specimen Cases A THESIS SUBMITTED TO THE GRADUATE FACULTY In partial fulfillment of the requirements For the degree of MASTER OF SCIENCE By Danielle Ross-Carr Edmond, Oklahoma 2017 DRUG IDENTIFICATION AND CONFIRMATION BY LC/MS-MS iii Acknowledgements I would like to express my appreciation to the Oklahoma State Bureau of Investigation Forensic Science Center and Andrea Swiech for allowing me the opportunity to complete this project and providing all necessary materials. A special thank you to Robert Weston for guiding me through the validation requirements and taking the time to work with me every step of the way. Thank you to Melissa Windham and Kourtney Heard for assisting with the required extractions and to Matt Stillwell for reviewing the completed data. To my committee chair, Dr. Thomas Jourdan, I would like to thank you for your support and guidance throughout the entirety of this project and for your feedback during the writing process. I would like to acknowledge my committee members, Dr. Wayne Lord and Dr. John Bowen for challenging me to think critically and preparing me to defend my project. To my family, friends, and co-workers, I would like to express my gratitude for all of your support and encouragement through this entire process. I cannot say thank you enough for your understanding and constant reassurance that I would make it to this point. Finally, to my wife, Kayla, for your unwavering support in everything that I do. -

MMC International BV
M.M.C. International Steroid Substances Steroid Test A Colour Steroid Test B Colour Steroid Test B Colour with UV Light Stanozolol/ Oxandrolone Test Clenbuterol/ Oxymetholone Test Ephedrine Test Alfadolone Orange Yellow Nil - - - Androsterone Orange Yellow White - - - Beclometasone Brown–yellow Orange Nil - - - Betamethasone Orange–brown Pink–Orange Nil - - - Boldenone Base (Equipoise, Ganabol) (pure powder) Warm red after 2 min. Dark Orange after 2 min. Bright Light Orange - - - Boldenone Undecanoate (oil) Dark brownish-red Dark Red Bright Light Orange - - - Boldenone Undecylenate (oil) Orange - Light Brown Dark Orange → Brown Bright Light Orange-Yellow - - - Carbenoxolone (CBX) Orange Yellow Yellow - - - Cholesterol Violet Orange White - - - Clenbuterol (Spiropent, Ventipulmin) - - - - Purple - Dark brown with yellow-green on the Dark brown with yellow-green on the Clomiphene (Androxal, Clomid, Omifin) Nil Dark brown to black No reaction Dark brown to black sides of the ampoule sides of the ampoule Cortisone Orange Yellow Green - - - Desoxycortone Blue–black Yellow Yellow - - - Dexamethasone Yellow Orange–pink Nil - - - Dienestrol Yellow Orange–red Nil - - - Diethylstilbestrol (DES) Orange (→yellow–green) Nil - - - Dimethisterone Brown–green Orange–red Yellow - - - Drostanolone Propionate (Masteron) (oil) Bright green Yellow-Orange Orange - - - Dydrogesterone (Duphaston) - Orange Green-Yellow - - - Enoxolone Orange Yellow Green-Yellow - - - Ephedrine (also for Pseudo- and Nor-Ephedrine) - - - - - Orange Estradiol (Oestradiol) Orange -

Toiminta Unita on Ulla La Mungukurti |
TOIMINTAUNITA USON 20180071390A1ULLA LA MUNGUKURTI | ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2018/ 0071390 A1 PATEL et al. (43 ) Pub . Date : Mar . 15 , 2018 ( 54 ) COMPOSITIONS OF PHARMACEUTICAL A61K 9 / 06 (2006 .01 ) ACTIVES CONTAINING DIETHYLENE A61K 9 /00 (2006 .01 ) GLYCOL MONOETHYL ETHER OR OTHER A61K 31 /573 ( 2006 .01 ) ALKYL DERIVATIVES A61K 31/ 565 ( 2006 .01 ) A61K 31/ 4439 ( 2006 . 01 ) ( 71 ) Applicant : THEMIS MEDICARE LIMITED , A61K 31 / 167 ( 2006 . 01 ) Mumbai (IN ) A61K 31 / 57 (2006 . 01) (52 ) U . S . CI. (72 ) Inventors : Dinesh Shantilal PATEL , Mumbai CPC .. .. .. A61K 47 / 10 ( 2013 . 01 ) ; A61K 9 /4858 ( IN ) ; Sachin Dinesh PATEL , Mumbai ( 2013 .01 ) ; A61K 9 /08 ( 2013 .01 ) ; A61K 9 / 06 ( IN ) ; Shashikant Prabhudas ( 2013 .01 ) ; A61K 9 / 0014 ( 2013 .01 ) ; A61K KURANI, Mumbai ( IN ) ; Madhavlal 31/ 573 ( 2013 .01 ) ; A61K 31 /57 ( 2013 .01 ) ; Govindlal PATEL , Mumbai ( IN ) A61K 31/ 565 ( 2013 .01 ) ; A61K 31 /4439 (73 ) Assignee : THEMIS MEDICARE LIMITED , ( 2013 .01 ) ; A61K 31/ 167 ( 2013 .01 ) ; A61K Mumbai (IN ) 9 /0048 ( 2013 .01 ) ; A61K 9 /0019 (2013 .01 ) ( 57 ) ABSTRACT (21 ) Appl. No .: 15 / 801, 390 The present invention relates to pharmaceutical composi tions of various pharmaceutical actives, especially lyophilic ( 22 ) Filed : Nov . 2 , 2017 and hydrophilic actives containing Diethylene glycol mono ethyl ether or other alkyl derivatives thereof as a primary Related U . S . Application Data vehicle and /or to pharmaceutical compositions utilizing (62 ) Division of application No. 14 /242 , 973 , filed on Apr. Diethylene glycol monoethyl ether or other alkyl derivatives 2 , 2014 , now Pat. No. 9 , 827 ,315 . -

(12) Patent Application Publication (10) Pub. No.: US 2014/0296.191 A1 PATEL Et Al
US 20140296.191A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0296.191 A1 PATEL et al. (43) Pub. Date: Oct. 2, 2014 (54) COMPOSITIONS OF PHARMACEUTICAL (52) U.S. Cl. ACTIVES CONTAINING DETHYLENE CPC ............... A61K 47/10 (2013.01); A61 K9/0019 GLYCOL MONOETHYLETHER OR OTHER (2013.01); A61 K9/0048 (2013.01); A61 K ALKYL DERVATIVES 45/06 (2013.01) USPC ........... 514/167: 514/177; 514/178: 514/450; (71) Applicant: THEMIS MEDICARE LIMITED, 514/334: 514/226.5: 514/449; 514/338; Mumbai (IN) 514/256; 514/570; 514/179; 514/174: 514/533; (72) Inventors: Dinesh Shantilal PATEL, Mumbai (IN); 514/629; 514/619 Sachin Dinesh PATEL, Mumbai (IN); Shashikant Prabhudas KURANI, Mumbai (IN); Madhavlal Govindlal (57) ABSTRACT PATEL, Mumbai (IN) (73) Assignee: THEMIS MEDICARE LIMITED, The present invention relates to pharmaceutical compositions Mumbai (IN) of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl (21) Appl. No.: 14/242,973 ether or other alkyl derivatives thereofas a primary vehicle and/or to pharmaceutical compositions utilizing Diethylene (22) Filed: Apr. 2, 2014 glycol monoethyl ether or other alkyl derivatives thereofas a primary vehicle or as a solvent system in preparation of Such (30) Foreign Application Priority Data pharmaceutical compositions. The pharmaceutical composi Apr. 2, 2013 (IN) ......................... 1287/MUMA2013 tions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formu Publication Classification lations containing such pharmaceutical actives and are Suit able for use as injectables for intravenous and intramuscular (51) Int. Cl. administration, as well as for use as a preformed solution/ A647/ (2006.01) liquid for filling in and preparation of capsules, tablets, nasal A6 IK 45/06 (2006.01) sprays, gargles, dermal applications, gels, topicals, liquid oral A6 IK9/00 (2006.01) dosage forms and other dosage forms. -

SEAC) Held on 01/10/2019 at Committee Room, Gujarat Pollution Control Board, Sector 10A,Gandhinagar
Minutes of the 554 th meeting ofthe State Level Expert Appraisal Committee (SEAC) held on 01/10/2019 at Committee Room, Gujarat Pollution Control Board, Sector 10A,Gandhinagar. The 554 th meeting of the State Level Expert Appraisal Committee (SEAC) was held on 1st October 2019 at Committee Room, Gujarat Pollution Control Board, Sector 10A, Gandhinagar. Following members attended the meeting: 1. Dr. Dinesh Misra, Chairman, SEAC 2. Shri S. C. Srivastav, Vice Chairman, SEAC 3. Shri Rajesh. I. Shah, Member, SEAC 4. Shri V. N. Patel, Member, SEAC 5. Dr. V. K. Jain, Member, SEAC 6. Shri A. K. Mule, Member, SEAC The regular agenda of Appraisal, Screening & Scoping/ToR, Reconsideration cases were taken up. The Committee considered the applications made by project proponents, additional details submitted as required by the SEAC/SEIAA and details furnished in the Form-1, PFR, EIA-EMP reports. Proposal No. Name and Address of the Unit Remarks 04 SIA/GJ/IND2/29059/2017 M/s. Dev Dyechem Industries EC – Plot No. C-1/324, Phase-I & II GIDC – Reconsideration Naroda, Ahmedabad. • PP remained absent in the EC – Appraisal presentation held on 01/10/2019. • After detailed discussion, Committee decided to defer the proposal and consider the same in one of the upcoming meeting of SEAC. 05 SIA/GJ/IND2/29678/2018 M/s. Apex Pharma EC – C-298/2/4, Saykha Industrial Estate, Reconsideration Saykha, Ta- Vagra, Dist -Bharuch. Category of the unit: 5(f) Project status: New • Project proponent (PP) submitted online application vide no. SIA/GJ/IND2/29678/2018 dated 30/04/19 for obtaining Environmental Clearance. -

Methiopropamine (MPA) Critical Review Report Agenda Item 4.8
Methiopropamine (MPA) Critical Review Report Agenda Item 4.8 Expert Committee on Drug Dependence Thirty-eighth Meeting Geneva, 14-18 November 2016 38th ECDD (2016) Agenda item 4.8 MPA Page 2 of 21 38th ECDD (2016) Agenda item 4.8 MPA Contents Acknowledgements .......................................................................................................................... 5 Summary .......................................................................................................................................... 6 1. Substance identification .................................................................................................... 7 A. International Nonproprietary Name (INN) ........................................................................ 7 B. Chemical Abstract Service (CAS) Registry Number .......................................................... 7 C. Other Chemical Names ...................................................................................................... 7 D. Trade Names ...................................................................................................................... 7 E. Street Names ...................................................................................................................... 7 F. Physical Appearance .......................................................................................................... 7 G. WHO Review History ......................................................................................................... 7 2. -

Alcohol and Drug Abuse Subchapter 9 Regulated Drug Rule 1.0 Authority
Chapter 8 – Alcohol and Drug Abuse Subchapter 9 Regulated Drug Rule 1.0 Authority This rule is established under the authority of 18 V.S.A. §§ 4201 and 4202 which authorizes the Vermont Board of Health to designate regulated drugs for the protection of public health and safety. 2.0 Purpose This rule designates drugs and other chemical substances that are illegal or judged to be potentially fatal or harmful for human consumption unless prescribed and dispensed by a professional licensed to prescribe or dispense them, and used in accordance with the prescription. The rule restricts the possession of certain drugs above a specified quantity. The rule also establishes benchmark unlawful dosages for certain drugs to provide a baseline for use by prosecutors to seek enhanced penalties for possession of higher quantities of the drug in accordance with multipliers found at 18 V.S.A. § 4234. 3.0 Definitions 3.1 “Analog” means one of a group of chemical components similar in structure but different with respect to elemental composition. It can differ in one or more atoms, functional groups or substructures, which are replaced with other atoms, groups or substructures. 3.2 “Benchmark Unlawful Dosage” means the quantity of a drug commonly consumed over a twenty-four hour period for any therapeutic purpose, as established by the manufacturer of the drug. Benchmark Unlawful dosage is not a medical or pharmacologic concept with any implication for medical practice. Instead, it is a legal concept established only for the purpose of calculating penalties for improper sale, possession, or dispensing of drugs pursuant to 18 V.S.A. -

Single Exposure to the Cathinones MDPV and Α-PVP Alters Molecular Markers of Neuroplasticity in the Adult Mouse Brain
International Journal of Molecular Sciences Article Single Exposure to the Cathinones MDPV and α-PVP Alters Molecular Markers of Neuroplasticity in the Adult Mouse Brain Lucia Caffino 1 , Francesca Mottarlini 1 , Sabrine Bilel 2, Giorgia Targa 1 , Micaela Tirri 2 , Coralie Maggi 1, Matteo Marti 2,3,† and Fabio Fumagalli 1,*,† 1 Department of Pharmacological and Biomolecular Sciences, Università degli Studi di Milano, Via Balzaretti 9, 20133 Milano, Italy; lucia.caffi[email protected] (L.C.); [email protected] (F.M.); [email protected] (G.T.); [email protected] (C.M.) 2 Section of Legal Medicine and LTTA Center, Department of Translational Medicine, University of Ferrara, 44121 Ferrara, Italy; [email protected] (S.B.); [email protected] (M.T.); [email protected] (M.M.) 3 Collaborative Center for the Italian National Early Warning System, Department of Anti-Drug Policies, Presidency of the Council of Ministers, 44121 Ferrara, Italy * Correspondence: [email protected]; Tel.: +39-02-50318298 † Fumagalli and Matteo Marti share the seniorship. Abstract: Synthetic cathinones have gained popularity among young drug users and are widely used in the clandestine market. While the cathinone-induced behavioral profile has been extensively investigated, information on their neuroplastic effects is still rather fragmentary. Accordingly, we have exposed male mice to a single injection of MDPV and α-PVP and sacrificed the animals at different time points (i.e., 30 min, 2 h, and 24 h) to have a rapid readout of the effect of these psychostimulants on neuroplasticity in the frontal lobe and hippocampus, two reward-related brain regions. -

NEWS 02 2020 ENG.Qxp Layout 1
Polymers and fluorescence Balance of power 360° drinking water analysis trilogy Fluorescence spectroscopy LCMS-8060NX: performance of industrial base polymers and robustness without Automatic, simultaneous and compromising sensitivity rapid analysis of pesticides and speed CONTENT APPLICATION »Plug und Play« disease screening solution? – The MALDI-8020 in screening for Sickle Cell Disease 4 Customized software solutions for any measurement – Macro programming for Shimadzu UV-Vis and FTIR 8 Ensuring steroid-free food supplements – Identification of steroids in pharmaceuticals and food supplements with LCMS-8045 11 MSn analysis of nonderivatized and Mtpp-derivatized peptides – Two recent studies applying LCMS-IT-TOF instruments 18 Polymers and fluorescence – Part 2: How much fluorescence does a polymer show during quality control? 26 PRODUCTS The balance of power – LCMS-8060NX balances enhanced performance and robustness 7 360° drinking water analysis: Episode 2 – Automatic, simul- taneous and rapid analysis of pesticides in drinking water by online SPE and UHPLC-MS/MS 14 Versatile testing tool for the automotive industry – Enrico Davoli with the PESI-MS system (research-use only [RUO] instrument) New HMV-G3 Series 17 No more headaches! A guide to choosing the perfect C18 column 22 Validated method for monoclonal antibody drugs – Assessment of the nSMOL methodology in Global solution through the validation of bevacizumab in human serum 24 global collaboration LATEST NEWS Global solution through global collaboration – Shimadzu Cancer diagnosis: -

2021 SB 1738 by Senator Brodeur 9-01008B-21 20211738__ Page 1 of 34 CODING
Florida Senate - 2021 SB 1738 By Senator Brodeur 9-01008B-21 20211738__ 1 A bill to be entitled 2 An act relating to controlled substances; amending s. 3 893.03, F.S.; excluding from Schedule I cannabis if it 4 is contained within a pharmaceutical product approved 5 by the United States Food and Drug Administration; 6 providing an effective date. 7 8 Be It Enacted by the Legislature of the State of Florida: 9 10 Section 1. Paragraph (c) of subsection (1) of section 11 893.03, Florida Statutes, is amended to read: 12 893.03 Standards and schedules.—The substances enumerated 13 in this section are controlled by this chapter. The controlled 14 substances listed or to be listed in Schedules I, II, III, IV, 15 and V are included by whatever official, common, usual, 16 chemical, trade name, or class designated. The provisions of 17 this section shall not be construed to include within any of the 18 schedules contained in this section any excluded drugs listed 19 within the purview of 21 C.F.R. s. 1308.22, styled “Excluded 20 Substances”; 21 C.F.R. s. 1308.24, styled “Exempt Chemical 21 Preparations”; 21 C.F.R. s. 1308.32, styled “Exempted 22 Prescription Products”; or 21 C.F.R. s. 1308.34, styled “Exempt 23 Anabolic Steroid Products.” 24 (1) SCHEDULE I.—A substance in Schedule I has a high 25 potential for abuse and has no currently accepted medical use in 26 treatment in the United States and in its use under medical 27 supervision does not meet accepted safety standards. -
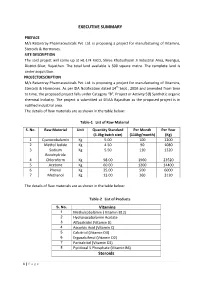
EXECUTIVE SUMMARY Steroids
EXECUTIVE SUMMARY PREFACE M/s Ratantray Pharmaceuticals Pvt. Ltd. is proposing a project for manufacturing of Vitamins, Steroids & Hormones. SITE DESCRIPTION The said project will come up at H1-174 RIICO, Shree Khatushyam Ji Industrial Area, Reengus, District-Sikar, Rajasthan. The total land available is 500 square metre. The complete land is under acquisition. PROJECTDESCRIPTION M/s Ratantray Pharmaceuticals Pvt. Ltd. is proposing a project for manufacturing of Vitamins, Steroids & Hormones. As per EIA Notification dated 14th Sept., 2006 and amended from time to time, the proposed project falls under Category “B”, Project or Activity 5(f) Synthetic organic chemical Industry. The project is submitted at SEIAA Rajasthan as the proposed project is in notified industrial area. The details of Raw materials are as shown in the table below: Table-1 List of Raw Material S. No. Raw Material Unit Quantity Standard Per Month Per Year (5.0kg batch size) (110kg/month) (Kg) 1 Cyanocobalamin Kg 5.00 100 1200 2 Methyl Iodide Kg 4.50 90 1080 3 Sodium Kg 5.50 110 1320 Borohydride 4 Chloroform Kg 98.00 1960 23520 5 Acetone Kg 60.00 1200 14400 6 Phenol Kg 25.00 500 6000 7 Methanol Kg 13.00 260 3120 The details of Raw materials are as shown in the table below: Table-2 List of Products S. No. Vitamins 1 Methylcobalamin ( Vitamin B12) 2 Hydroxocobalamin Acetate 3 Alfacalcidol (Vitamin D) 4 Ascorbic Acid (Vitamin C) 5 Calcitriol (Vitamin D3) 6 Ergocalciferol (Vitamin D2) 7 Paricalcitol (Vitamin D2) 8 Pyridoxal 5 Phosphate (Vitamin B6) Steroids 1 | Page 1 Beclomethasone -

Injectable Steroids Oral Steroids Post Cycle Therapy Fat Burners Manufacturers Contact Us
Blog Shopping Cart Wishlist My Account Checkout Search $0.00 Menu STEROIDS SHOP INJECTABLE STEROIDS ORAL STEROIDS POST CYCLE THERAPY FAT BURNERS MANUFACTURERS CONTACT US Injectable Steroids Shop Injectable Steroids SORT BY POPULARITY Search SOLD OUT INJECTABLE STEROIDS Deca Durabolin Equipoise HGH Masteron NPP INJECTABLE STEROIDS Parabolan Androxine $90.00 Primobolan Depot Sustanon 250 Testosterone Cypionate Testosterone Enanthate Testosterone Propionate Testosterone Suspension Trenbolone Acetate Trenbolone Enanthate Trenbolone Suspension Tri Tren Winstrol Depot ORAL STEROIDS INJECTABLE STEROIDS Anadrol Aquaviron Anavar $66.00 Dianabol Halotestin Methyltrienolone Moda됍nil Primobolan Proviron Superdrol Testosterone Undecanoate Turinabol Winstrol POST CYCLE THERAPY Anastrozole EQUIPOISE Boldebolin Cabergoline $60.00 Clomid Exemestane HCG HMG Letrozole Nolvadex FAT BURNERS Clenbuterol Cytomel T3 MANUFACTURERS EQUIPOISE Alpha Pharma Boldebolin (vial) Eminence Labs $55.00 Maxtreme Pharma SOLD OUT EQUIPOISE BoldePrime $69.00 EQUIPOISE Bold-Max $73.00 SOLD OUT EQUIPOISE Bold-One $39.00 SOLD OUT INJECTABLE STEROIDS CypoPrime $43.00 SOLD OUT DECA DURABOLIN DecaPrime $54.00 INJECTABLE STEROIDS Alphabolin $62.00 INJECTABLE STEROIDS Alphabolin (vial) $125.00 SOLD OUT INJECTABLE STEROIDS EnaPrime $43.00 HGH Eutropin 4 IU $55.00 HGH Humatrope $550.00 INJECTABLE STEROIDS Induject-250 $59.00 INJECTABLE STEROIDS Induject-250 (vial) $59.00 SOLD OUT INJECTABLE STEROIDS DrostoPrime $72.00 INJECTABLE STEROIDS