Steroids - Drugs & Intermediates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

(12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness Et Al
USOO6264,917B1 (12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness et al. (45) Date of Patent: Jul. 24, 2001 (54) TARGETED ULTRASOUND CONTRAST 5,733,572 3/1998 Unger et al.. AGENTS 5,780,010 7/1998 Lanza et al. 5,846,517 12/1998 Unger .................................. 424/9.52 (75) Inventors: Jo Klaveness; Pál Rongved; Dagfinn 5,849,727 12/1998 Porter et al. ......................... 514/156 Lovhaug, all of Oslo (NO) 5,910,300 6/1999 Tournier et al. .................... 424/9.34 FOREIGN PATENT DOCUMENTS (73) Assignee: Nycomed Imaging AS, Oslo (NO) 2 145 SOS 4/1994 (CA). (*) Notice: Subject to any disclaimer, the term of this 19 626 530 1/1998 (DE). patent is extended or adjusted under 35 O 727 225 8/1996 (EP). U.S.C. 154(b) by 0 days. WO91/15244 10/1991 (WO). WO 93/20802 10/1993 (WO). WO 94/07539 4/1994 (WO). (21) Appl. No.: 08/958,993 WO 94/28873 12/1994 (WO). WO 94/28874 12/1994 (WO). (22) Filed: Oct. 28, 1997 WO95/03356 2/1995 (WO). WO95/03357 2/1995 (WO). Related U.S. Application Data WO95/07072 3/1995 (WO). (60) Provisional application No. 60/049.264, filed on Jun. 7, WO95/15118 6/1995 (WO). 1997, provisional application No. 60/049,265, filed on Jun. WO 96/39149 12/1996 (WO). 7, 1997, and provisional application No. 60/049.268, filed WO 96/40277 12/1996 (WO). on Jun. 7, 1997. WO 96/40285 12/1996 (WO). (30) Foreign Application Priority Data WO 96/41647 12/1996 (WO). -

MMC International BV
M.M.C. International Steroid Substances Steroid Test A Colour Steroid Test B Colour Steroid Test B Colour with UV Light Stanozolol/ Oxandrolone Test Clenbuterol/ Oxymetholone Test Ephedrine Test Alfadolone Orange Yellow Nil - - - Androsterone Orange Yellow White - - - Beclometasone Brown–yellow Orange Nil - - - Betamethasone Orange–brown Pink–Orange Nil - - - Boldenone Base (Equipoise, Ganabol) (pure powder) Warm red after 2 min. Dark Orange after 2 min. Bright Light Orange - - - Boldenone Undecanoate (oil) Dark brownish-red Dark Red Bright Light Orange - - - Boldenone Undecylenate (oil) Orange - Light Brown Dark Orange → Brown Bright Light Orange-Yellow - - - Carbenoxolone (CBX) Orange Yellow Yellow - - - Cholesterol Violet Orange White - - - Clenbuterol (Spiropent, Ventipulmin) - - - - Purple - Dark brown with yellow-green on the Dark brown with yellow-green on the Clomiphene (Androxal, Clomid, Omifin) Nil Dark brown to black No reaction Dark brown to black sides of the ampoule sides of the ampoule Cortisone Orange Yellow Green - - - Desoxycortone Blue–black Yellow Yellow - - - Dexamethasone Yellow Orange–pink Nil - - - Dienestrol Yellow Orange–red Nil - - - Diethylstilbestrol (DES) Orange (→yellow–green) Nil - - - Dimethisterone Brown–green Orange–red Yellow - - - Drostanolone Propionate (Masteron) (oil) Bright green Yellow-Orange Orange - - - Dydrogesterone (Duphaston) - Orange Green-Yellow - - - Enoxolone Orange Yellow Green-Yellow - - - Ephedrine (also for Pseudo- and Nor-Ephedrine) - - - - - Orange Estradiol (Oestradiol) Orange -

Toiminta Unita on Ulla La Mungukurti |
TOIMINTAUNITA USON 20180071390A1ULLA LA MUNGUKURTI | ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2018/ 0071390 A1 PATEL et al. (43 ) Pub . Date : Mar . 15 , 2018 ( 54 ) COMPOSITIONS OF PHARMACEUTICAL A61K 9 / 06 (2006 .01 ) ACTIVES CONTAINING DIETHYLENE A61K 9 /00 (2006 .01 ) GLYCOL MONOETHYL ETHER OR OTHER A61K 31 /573 ( 2006 .01 ) ALKYL DERIVATIVES A61K 31/ 565 ( 2006 .01 ) A61K 31/ 4439 ( 2006 . 01 ) ( 71 ) Applicant : THEMIS MEDICARE LIMITED , A61K 31 / 167 ( 2006 . 01 ) Mumbai (IN ) A61K 31 / 57 (2006 . 01) (52 ) U . S . CI. (72 ) Inventors : Dinesh Shantilal PATEL , Mumbai CPC .. .. .. A61K 47 / 10 ( 2013 . 01 ) ; A61K 9 /4858 ( IN ) ; Sachin Dinesh PATEL , Mumbai ( 2013 .01 ) ; A61K 9 /08 ( 2013 .01 ) ; A61K 9 / 06 ( IN ) ; Shashikant Prabhudas ( 2013 .01 ) ; A61K 9 / 0014 ( 2013 .01 ) ; A61K KURANI, Mumbai ( IN ) ; Madhavlal 31/ 573 ( 2013 .01 ) ; A61K 31 /57 ( 2013 .01 ) ; Govindlal PATEL , Mumbai ( IN ) A61K 31/ 565 ( 2013 .01 ) ; A61K 31 /4439 (73 ) Assignee : THEMIS MEDICARE LIMITED , ( 2013 .01 ) ; A61K 31/ 167 ( 2013 .01 ) ; A61K Mumbai (IN ) 9 /0048 ( 2013 .01 ) ; A61K 9 /0019 (2013 .01 ) ( 57 ) ABSTRACT (21 ) Appl. No .: 15 / 801, 390 The present invention relates to pharmaceutical composi tions of various pharmaceutical actives, especially lyophilic ( 22 ) Filed : Nov . 2 , 2017 and hydrophilic actives containing Diethylene glycol mono ethyl ether or other alkyl derivatives thereof as a primary Related U . S . Application Data vehicle and /or to pharmaceutical compositions utilizing (62 ) Division of application No. 14 /242 , 973 , filed on Apr. Diethylene glycol monoethyl ether or other alkyl derivatives 2 , 2014 , now Pat. No. 9 , 827 ,315 . -

(12) Patent Application Publication (10) Pub. No.: US 2014/0296.191 A1 PATEL Et Al
US 20140296.191A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0296.191 A1 PATEL et al. (43) Pub. Date: Oct. 2, 2014 (54) COMPOSITIONS OF PHARMACEUTICAL (52) U.S. Cl. ACTIVES CONTAINING DETHYLENE CPC ............... A61K 47/10 (2013.01); A61 K9/0019 GLYCOL MONOETHYLETHER OR OTHER (2013.01); A61 K9/0048 (2013.01); A61 K ALKYL DERVATIVES 45/06 (2013.01) USPC ........... 514/167: 514/177; 514/178: 514/450; (71) Applicant: THEMIS MEDICARE LIMITED, 514/334: 514/226.5: 514/449; 514/338; Mumbai (IN) 514/256; 514/570; 514/179; 514/174: 514/533; (72) Inventors: Dinesh Shantilal PATEL, Mumbai (IN); 514/629; 514/619 Sachin Dinesh PATEL, Mumbai (IN); Shashikant Prabhudas KURANI, Mumbai (IN); Madhavlal Govindlal (57) ABSTRACT PATEL, Mumbai (IN) (73) Assignee: THEMIS MEDICARE LIMITED, The present invention relates to pharmaceutical compositions Mumbai (IN) of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl (21) Appl. No.: 14/242,973 ether or other alkyl derivatives thereofas a primary vehicle and/or to pharmaceutical compositions utilizing Diethylene (22) Filed: Apr. 2, 2014 glycol monoethyl ether or other alkyl derivatives thereofas a primary vehicle or as a solvent system in preparation of Such (30) Foreign Application Priority Data pharmaceutical compositions. The pharmaceutical composi Apr. 2, 2013 (IN) ......................... 1287/MUMA2013 tions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formu Publication Classification lations containing such pharmaceutical actives and are Suit able for use as injectables for intravenous and intramuscular (51) Int. Cl. administration, as well as for use as a preformed solution/ A647/ (2006.01) liquid for filling in and preparation of capsules, tablets, nasal A6 IK 45/06 (2006.01) sprays, gargles, dermal applications, gels, topicals, liquid oral A6 IK9/00 (2006.01) dosage forms and other dosage forms. -

SEAC) Held on 01/10/2019 at Committee Room, Gujarat Pollution Control Board, Sector 10A,Gandhinagar
Minutes of the 554 th meeting ofthe State Level Expert Appraisal Committee (SEAC) held on 01/10/2019 at Committee Room, Gujarat Pollution Control Board, Sector 10A,Gandhinagar. The 554 th meeting of the State Level Expert Appraisal Committee (SEAC) was held on 1st October 2019 at Committee Room, Gujarat Pollution Control Board, Sector 10A, Gandhinagar. Following members attended the meeting: 1. Dr. Dinesh Misra, Chairman, SEAC 2. Shri S. C. Srivastav, Vice Chairman, SEAC 3. Shri Rajesh. I. Shah, Member, SEAC 4. Shri V. N. Patel, Member, SEAC 5. Dr. V. K. Jain, Member, SEAC 6. Shri A. K. Mule, Member, SEAC The regular agenda of Appraisal, Screening & Scoping/ToR, Reconsideration cases were taken up. The Committee considered the applications made by project proponents, additional details submitted as required by the SEAC/SEIAA and details furnished in the Form-1, PFR, EIA-EMP reports. Proposal No. Name and Address of the Unit Remarks 04 SIA/GJ/IND2/29059/2017 M/s. Dev Dyechem Industries EC – Plot No. C-1/324, Phase-I & II GIDC – Reconsideration Naroda, Ahmedabad. • PP remained absent in the EC – Appraisal presentation held on 01/10/2019. • After detailed discussion, Committee decided to defer the proposal and consider the same in one of the upcoming meeting of SEAC. 05 SIA/GJ/IND2/29678/2018 M/s. Apex Pharma EC – C-298/2/4, Saykha Industrial Estate, Reconsideration Saykha, Ta- Vagra, Dist -Bharuch. Category of the unit: 5(f) Project status: New • Project proponent (PP) submitted online application vide no. SIA/GJ/IND2/29678/2018 dated 30/04/19 for obtaining Environmental Clearance. -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2019 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

PHARMACEUTICAL APPENDIX to the HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2008) (Rev
Harmonized Tariff Schedule of the United States (2008) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2008) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM GADOCOLETICUM 280776-87-6 ABAFUNGIN 129639-79-8 ACIDUM LIDADRONICUM 63132-38-7 ABAMECTIN 65195-55-3 ACIDUM SALCAPROZICUM 183990-46-7 ABANOQUIL 90402-40-7 ACIDUM SALCLOBUZICUM 387825-03-8 ABAPERIDONUM 183849-43-6 ACIFRAN 72420-38-3 ABARELIX 183552-38-7 ACIPIMOX 51037-30-0 ABATACEPTUM 332348-12-6 ACITAZANOLAST 114607-46-4 ABCIXIMAB 143653-53-6 ACITEMATE 101197-99-3 ABECARNIL 111841-85-1 ACITRETIN 55079-83-9 ABETIMUSUM 167362-48-3 ACIVICIN 42228-92-2 ABIRATERONE 154229-19-3 ACLANTATE 39633-62-0 ABITESARTAN 137882-98-5 ACLARUBICIN 57576-44-0 ABLUKAST 96566-25-5 ACLATONIUM NAPADISILATE 55077-30-0 ABRINEURINUM 178535-93-8 ACODAZOLE 79152-85-5 ABUNIDAZOLE 91017-58-2 ACOLBIFENUM 182167-02-8 ACADESINE 2627-69-2 ACONIAZIDE 13410-86-1 ACAMPROSATE -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2011) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2011) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

NEWS 02 2020 ENG.Qxp Layout 1
Polymers and fluorescence Balance of power 360° drinking water analysis trilogy Fluorescence spectroscopy LCMS-8060NX: performance of industrial base polymers and robustness without Automatic, simultaneous and compromising sensitivity rapid analysis of pesticides and speed CONTENT APPLICATION »Plug und Play« disease screening solution? – The MALDI-8020 in screening for Sickle Cell Disease 4 Customized software solutions for any measurement – Macro programming for Shimadzu UV-Vis and FTIR 8 Ensuring steroid-free food supplements – Identification of steroids in pharmaceuticals and food supplements with LCMS-8045 11 MSn analysis of nonderivatized and Mtpp-derivatized peptides – Two recent studies applying LCMS-IT-TOF instruments 18 Polymers and fluorescence – Part 2: How much fluorescence does a polymer show during quality control? 26 PRODUCTS The balance of power – LCMS-8060NX balances enhanced performance and robustness 7 360° drinking water analysis: Episode 2 – Automatic, simul- taneous and rapid analysis of pesticides in drinking water by online SPE and UHPLC-MS/MS 14 Versatile testing tool for the automotive industry – Enrico Davoli with the PESI-MS system (research-use only [RUO] instrument) New HMV-G3 Series 17 No more headaches! A guide to choosing the perfect C18 column 22 Validated method for monoclonal antibody drugs – Assessment of the nSMOL methodology in Global solution through the validation of bevacizumab in human serum 24 global collaboration LATEST NEWS Global solution through global collaboration – Shimadzu Cancer diagnosis: -
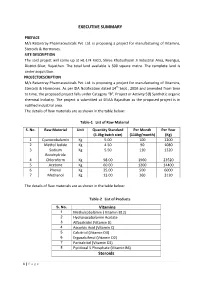
EXECUTIVE SUMMARY Steroids
EXECUTIVE SUMMARY PREFACE M/s Ratantray Pharmaceuticals Pvt. Ltd. is proposing a project for manufacturing of Vitamins, Steroids & Hormones. SITE DESCRIPTION The said project will come up at H1-174 RIICO, Shree Khatushyam Ji Industrial Area, Reengus, District-Sikar, Rajasthan. The total land available is 500 square metre. The complete land is under acquisition. PROJECTDESCRIPTION M/s Ratantray Pharmaceuticals Pvt. Ltd. is proposing a project for manufacturing of Vitamins, Steroids & Hormones. As per EIA Notification dated 14th Sept., 2006 and amended from time to time, the proposed project falls under Category “B”, Project or Activity 5(f) Synthetic organic chemical Industry. The project is submitted at SEIAA Rajasthan as the proposed project is in notified industrial area. The details of Raw materials are as shown in the table below: Table-1 List of Raw Material S. No. Raw Material Unit Quantity Standard Per Month Per Year (5.0kg batch size) (110kg/month) (Kg) 1 Cyanocobalamin Kg 5.00 100 1200 2 Methyl Iodide Kg 4.50 90 1080 3 Sodium Kg 5.50 110 1320 Borohydride 4 Chloroform Kg 98.00 1960 23520 5 Acetone Kg 60.00 1200 14400 6 Phenol Kg 25.00 500 6000 7 Methanol Kg 13.00 260 3120 The details of Raw materials are as shown in the table below: Table-2 List of Products S. No. Vitamins 1 Methylcobalamin ( Vitamin B12) 2 Hydroxocobalamin Acetate 3 Alfacalcidol (Vitamin D) 4 Ascorbic Acid (Vitamin C) 5 Calcitriol (Vitamin D3) 6 Ergocalciferol (Vitamin D2) 7 Paricalcitol (Vitamin D2) 8 Pyridoxal 5 Phosphate (Vitamin B6) Steroids 1 | Page 1 Beclomethasone