Essentials of the SI Background Bibliography Introduction Some
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

The International System of Units (SI)
NAT'L INST. OF STAND & TECH NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 330 2001 Edition The International System of Units (SI) 4. Barry N. Taylor, Editor r A o o L57 330 2oOI rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -

Innovative Concepts and Technology for Railroad-Highway Grade
NOTICE This document is disseminated under the sponsorship of the Department of Transportation in the interest of information exchange. The United States Govern ment assumes no liability for its contents or use thereof. NOTICE The United States Government does not endorse pro ducts or manufacturers. Trade or manufacturers' names appear herein solely because they are con sidered essential to the object of this report. T ~chnical Repart Docum~ntotion Poge 1. RopOr! No. 12. G.y.,"~.", Acce",," No. 3. Reciplenl's Colalog No ;- FRA/ORD - 77/37 .II ~b -1-'1 ~~ ') - '?> I 4. Title and Subtitle 5. Reporr Dale INKOVATIVE CONCEPTS AND TECHNOLOGY FOR September 1977 I RAILROAD -HIGH1\'AY GRADE CROSSING MOTORIST 6. Pe,Iorrnlng Organ, 101 on Code' t WARNING SYSTEl>lS Vol. II: The Generation and ~ Analysis of Alternative Concepts 8. Pe,formlng Orgonl10tlon Reperr No. 7. Au1f-or's) : D.O. Peterson and D.S. Boyer DOT-TSC-FRA-76-l9.II 9. Per'OfrT\lng Orgar1l10tio... Name and Address 10. Wor ... Unl' No. (TRAIS) Tracor-Jitco, Inc. RR602/R7337 * I 11. Contract Or Granl No. i 1776 East Jefferson Street : DOT-TSC-842-2 Rockville MD 20852 , 13. l)'pe of Report and Per·cei Covered 12. Spon,oring Agency Name and Addren I U.S. Department of Transportation , Final Report : Federal Railroad Administration June 1974 -ylarch 1976 Office of Research and Development 14. SpanlGring A90ner Code Washington DC 20590 15. Supplemenlary Notes : U.S. Department of Transportation !*Under contract to: Transportation Systems Center I Kendall Square Cambridge t-IA 02142 I 16. Abstroct I This report describes the results of a study directed toward the I generation, analysis and evaluation of innovative conceptual and technical approahces to train-activated motorist warning systems for i use at railroad-highway grade crossings. -

Appendix K: Conversion of Units
Appendix K: Conversion of Units Each of the fractions below has the value of 1, i.e., numerator and denominator are equal. Hence multiplying any dimension by one of the fractions ͑and cancelling terms͒ does not change the value. Circular measure 0.01745 radians 57.30 degrees 9.55 rev/minute degree radian rad/s Linear measure 0.3048 m 3.281 ft 1.609 km 0.6214 statute mile 1.852 km 1.1516 statute mile 60 nautical miles ft m statute mile km nautical mile nautical mile degree at equator 2.54 cm 106 micron 1010 Angstro¨m 9.46 m 66 ft 100 link 6ft 1 league inch m m 10Ϫ15 light year chain chain fathom 3 statute miles Velocity 1.689 ft/s 1.15157 mile/hour 0.5148 m/s 1.853 km/hour knot knot knot knot Area 1028 barn 640 acres 1 section 2.471 acre 2.590 km2 100 hectare 0.4042 hectare 258.7 hectare m2 mile2 mile2 hectare mile2 km2 acre mile2 9mi2 5760 acre Gulf of Mexico ͑GOM͒ block GOM block Volume 3.785 liters 4.546 liters 7.4805 U.S. gallons 0.15899 m3 0.028 m3 159 liter U.S. gallon British gallon ft3 U.S. bbl ft3 U.S. bbl 1 acre ft 7758 bbl 5.61 ft3 1 U.S. bbl 42 U.S. gallons 1233 m3 1233.5 m3 acre ft U.S. bbl 0.159 m3 U.S. bbl acre ft Mass 2.2046 lb 0.4536 kg 1.120 short ton 1.102 short ton 0.9842 long ton kg lb long ton metric tonne metric tonne Pressure 1.01325 pascal 1 bar 29.92 inches of Hg 14.223 lb/inch2 1cmofHg 10Ϫ5 atmosphere 105 pascal atmosphere 104 kg/m2 1333 pascal 14.7 psi 1 newton/m2 0.06895 bar 703.07 kg/m2 0.1333 kPa 16.018 kg/m3 atmosphere pascal lb/inch2 lb/inch2 torr lb/ft3 0.069 bar 6.895 kilopascal 0.01014 atm psi psi kPa 420 421 Appendix J Normal hydrostatic pressure gradient 0.43–0.45 psi/ft 0.43–0.45 psi/ft 9.5–10.2 kPa/m 8.33–8.65 ppg EMW Work „Energy… 1055 joules 4186 joules 3600 joules 1.6020 joule 0.2930 watt/hour BTU kilocalorie watt hour 1019 electron volt BTU 0.948 BTU 1.055 kilojoule 107 erg 0.06895 bar BTU in 6 MCF gas kilojoule BTU joule lb/in2ϭpsi BTU in U.S. -

Guide for the Use of the International System of Units (SI)
NI3T PUBLICATIONS Nisr c National Institute of 034Sfi l A11107 Standards and Technology U.S. Department of Commerce Guide for the Use of the International System of Units (SI) NIST Special Publication 81 1 • 200S Edition Ambler Thompson and Barry N. Taylor Iqc 100 1 1.U57 l#811 I2OO8 |c.2 NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 81 1 , 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 85 pages (March 2008, 1 printing) CODEN: NSPUE3 Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French he Systeme International d'Unites), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. industry increase its competitiveness in the global marketplace. -

The International System of Units (SI)
The International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 330 2008 Edition Barry N. Taylor and Ambler Thompson, Editors NIST SPECIAL PUBLICATION 330 2008 EDITION THE INTERNATIONAL SYSTEM OF UNITS (SI) Editors: Barry N. Taylor Physics Laboratory Ambler Thompson Technology Services National Institute of Standards and Technology Gaithersburg, MD 20899 United States version of the English text of the eighth edition (2006) of the International Bureau of Weights and Measures publication Le Système International d’ Unités (SI) (Supersedes NIST Special Publication 330, 2001 Edition) Issued March 2008 U.S. DEPARTMENT OF COMMERCE, Carlos M. Gutierrez, Secretary NATIONAL INSTITUTE OF STANDARDS AND TECHNOLOGY, James Turner, Acting Director National Institute of Standards and Technology Special Publication 330, 2008 Edition Natl. Inst. Stand. Technol. Spec. Pub. 330, 2008 Ed., 96 pages (March 2008) CODEN: NSPUE2 WASHINGTON 2008 Foreword The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant system used in science, the SI is rapidly becoming the dominant measurement system used in international commerce. In recognition of this fact and the increasing global nature of the marketplace, the Omnibus Trade and Competitiveness Act of 1988, which changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. industry increase its competitiveness, designates “the metric system of measurement as the preferred system of weights and measures for United States trade and commerce.” The definitive international reference on the SI is a booklet published by the International Bureau of Weights and Measures (BIPM, Bureau International des Poids et Mesures) and often referred to as the BIPM SI Brochure. -

Guide .Or Metric Practice
GUIDE OR METRIC PRACTICE Internationally recognized conventions have been established for standard usage of SI units. Robert A. Nelson ROBERT NELSON is the author of the booklet SI: The International System of Units, 2nd ed. (American Association of Physics Teachers, College Park, Maryland, 1982). He is president of Satellite Engineering Research Corporation, a consulting firm in Bethesda, Maryland, and teaches in the department of aerospace engineering at the University of Maryland. he modernized metric system is known as the Système TInternational d’Unités (International System of Table 1. SI base units Units), with the international abbreviation SI. It is found- Quantity Unit ed on seven base units, listed in table 1, that by conven- Name Symbol tion are regarded as dimensionally independent. All other length meter m mass kilogram kg units are derived units, formed coherently by multiplying time second s and dividing units within the system without numerical electric current ampere A factors. Examples of derived units, including some with thermodynamic temperature kelvin K special names, are listed in table 2. The expression of amount of substance mole mol multiples and submultiples of SI units is facilitated luminous intensity candela cd through the use of the prefixes listed in table 3. SI obtains its international authority from the Meter Convention, signed in Paris by the delegates of 17 coun- Table 2. Examples of SI derived units tries, including the United States, on 20 May 1875, and Quantity Unit Special name Symbol Equivalent amended in 1921. Today 49 countries are members. The plane angle radian rad m/m=1 treaty established the Conférence Générale des Poids et solid angle steradian sr m22 /m =1 speed, velocity m/s Mesures (General Conference on Weights and Measures) acceleration m/s2 as the formal diplomatic body responsible for ratification angular velocity rad/s of new proposals related to metric units. -

On the Dimension Group of Classical Physics
JOURNAL OF RESEARCH of the National Bureau of Stondards - B. Mathematical Sciences Va l. 79B, Nos. 3 and 4, July- December 1975 On the Dimension Group of Classical Physics Chester H. Page Institute for Basic Standards, National Bureau of Standards, Washington, D.C. 20234 (May 15, 1975) The basic principles of the group properties of physical quantities are reviewed. The proble ms associated with the dimensions of angle and logarithm are solved by using functional equations, in stead of a nalytic expressions, for de fining functions of non numerical quantities. It is concluded that the neper and radian are related by Np= - j rad, so that when the radian is considered as a base unit . the neper becomes a derived unit, and is the 5I unit for logarithmic quantities. Key word s: Angle : dime nsion; logarithm: neper: radi an. 1. Introduction Theoreti cal physics and e ngineering are based on mathematical models of the phe nomena of nature, i.e., the relations among measurable physical entities are expressed by mathe matical equations. Scientists ori ginally considered these equations to be relati ons among numbers, with the numbers re presenting the measures of quantities expressed in terms of agreed-upon units. Maxwell was one of the first to realize that this inte rpretation of equations in science is sterile com pared to the quantity calculus concept. In the modern quantity equation vi ewpoint, the letter symbols in an equation re present mathe mati cal ele me nts, called quantities, which are in a one-to one correspondence with the measurable entities of the laboratory. -
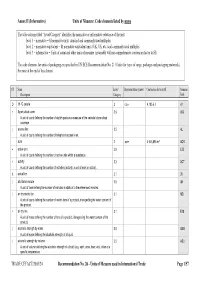
Units of Measure Used in International Trade Page 1/57 Annex II (Informative) Units of Measure: Code Elements Listed by Name
Annex II (Informative) Units of Measure: Code elements listed by name The table column titled “Level/Category” identifies the normative or informative relevance of the unit: level 1 – normative = SI normative units, standard and commonly used multiples level 2 – normative equivalent = SI normative equivalent units (UK, US, etc.) and commonly used multiples level 3 – informative = Units of count and other units of measure (invariably with no comprehensive conversion factor to SI) The code elements for units of packaging are specified in UN/ECE Recommendation No. 21 (Codes for types of cargo, packages and packaging materials). See note at the end of this Annex). ST Name Level/ Representation symbol Conversion factor to SI Common Description Category Code D 15 °C calorie 2 cal₁₅ 4,185 5 J A1 + 8-part cloud cover 3.9 A59 A unit of count defining the number of eighth-parts as a measure of the celestial dome cloud coverage. | access line 3.5 AL A unit of count defining the number of telephone access lines. acre 2 acre 4 046,856 m² ACR + active unit 3.9 E25 A unit of count defining the number of active units within a substance. + activity 3.2 ACT A unit of count defining the number of activities (activity: a unit of work or action). X actual ton 3.1 26 | additional minute 3.5 AH A unit of time defining the number of minutes in addition to the referenced minutes. | air dry metric ton 3.1 MD A unit of count defining the number of metric tons of a product, disregarding the water content of the product. -

Technical Standards Board Standard
TECHNICAL REV. STANDARDS TSB 003 MAY1999 BOARD Issued 1965-06 400 Commonwealth Drive, Warrendale, PA 15096-0001 STANDARD Revised 1999-05 Superseding TSB 003 JUN92 Submitted for recognition as an American National Standard Rules for SAE Use of SI (Metric) Units Foreword—SI denotes The International System of Units (Le Système International d’Unités). SI was established in 1960, under the Treaty of the Meter, by Resolutions and Recommendations of the General Conference on Weights and Measures (Conférence Générale des Poids et Mesures, CGPM) and the International Committee for Weights and Measures (Comité International des Poids et Mesures, CIPM) on The International System of Units. The abbreviation "SI" is used in all languages. In 1969, the SAE Board of Directors issued a directive that "SAE will include SI units in SAE Standards and other technical reports." During the ensuing several decades, SAE metric policy evolved and implementation progressed. The SAE’s current metric policy is, "Operating Boards shall not use any weights and measures system other than metric (SI), except when conversion is not practical, or where a conflicting world industry practice exists." Principal driving forces for SAE metrication were: worldwide movement to metric units; enactment of United States Federal metric legislation and the resultant national metrication activity; the international trend in industry and business throughout the world, and the growing international scope of SAE. Currently, the widespread, strong support for international standards harmonization is another key motivating factor in the global metrication movement. TSB 003 (formerly SAE J916) has been updated periodically, to reflect SAE metric policy evolution—as well as developments in the specific, formal content of SI; and in the correct, consistent usage and application of SI...which sometimes is referred to as "the modern version of the metric measurement system." The content of TSB 003 is consistent with international and U.S. -

Siunitx – a Comprehensive (Si) Units Package∗
siunitx – A comprehensive (si) units package∗ Joseph Wright† Released 2021-09-22 Contents 1 Introduction 3 2 siunitx for the impatient 3 3 Using the siunitx package 4 3.1 Numbers . 4 3.2 Angles . 5 3.3 Units . 6 3.4 Complex numbers and quantities . 7 3.5 The unit macros . 8 3.6 Unit abbreviations . 10 3.7 Creating new macros . 14 3.8 Tabular material . 15 4 Package control options 17 4.1 The key–value control system . 17 4.2 Printing . 18 4.3 Parsing numbers . 20 4.4 Post-processing numbers . 22 4.5 Printing numbers . 25 4.6 Lists, products and ranges . 28 4.7 Complex numbers . 31 4.8 Angles . 32 4.9 Creating units . 34 4.10 Using units . 35 4.11 Quantities . 38 4.12 Tabular material . 39 4.13 Locale options . 49 4.14 Preamble-only options . 49 5 Upgrading from version 2 49 6 Unit changes made by BIPM 51 ∗This file describes v3.0.31, last revised 2021-09-22. †E-mail: [email protected] 1 7 Localisation 52 8 Compatibility with other packages 52 9 Hints for using siunitx 52 9.1 Adjusting \litre and \liter ........................ 52 9.2 Ensuring text or math output . 53 9.3 Expanding content in tables . 53 9.4 Using siunitx with datatool .......................... 54 9.5 Using units in section headings and bookmarks . 55 9.6 A left-aligned column visually centred under a heading . 56 9.7 Regression tables . 57 9.8 Maximising performance . 58 9.9 Special considerations for the \kWh unit . -

Units and Dimensions
RaoApp-Cv3.qxd 12/18/03 5:46 PM Page 795 APPENDIX C Units and Dimensions In 1960, the International System of Units was given official status at the Eleventh General Conference on Weights and Measures held in Paris. This sys- tem of units is an expanded version of the rationalized meter–kilogram–second– ampere (MKSA) system of units and is based on six fundamental, or basic, units.The six basic units are the units of length, mass, time, current, temperature, and luminous intensity. The international unit of length is the meter. It is exactly 1,650,763.73 times the wavelength in vacuum of the radiation corresponding to the unper- turbed transition between the levels 2p10 and 5d5 of the atom of krypton-86, the orange-red line. The international unit of mass is the kilogram. It is the mass of the International Prototype Kilogram, which is a particular cylinder of platinum– iridium alloy preserved in a vault at Sèvres, France, by the International Bureau of Weights and Measures.The international unit of time is the second. It is equal to 9,192,631,770 times the period corresponding to the frequency of the transi- tion between the hyperfine levels F = 4, M = 0 and F = 3, M = 0 of the fun- 2 damental state S1 2 of the cesium-133 atom unperturbed by external fields. > To present the definition for the international unit of current, we first de- fine the newton, which is the unit of force, derived from the fundamental units meter, kilogram, and second in the following manner.