International System of Units Introduction SI Base Units SI Derived
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calculating Growing Degree Days by Jim Nugent District Horticulturist Michigan State University
Calculating Growing Degree Days By Jim Nugent District Horticulturist Michigan State University Are you calculating degree day accumulations and finding your values don't match the values being reported by MSU or your neighbor's electronic data collection unit? There is a logical reason! Three different methods are used to calculate degree days; i.e., 1) Averaging Method; 2) Baskerville-Emin (BE) Method; and 3) Electronic Real-time Data Collection. All methods attempt to calculate the heat accumulation above a minimum threshold temperature, often referred to the base temperature. Once both the daily maximum and minimum temperatures get above the minimum threshold temperature, (i.e., base temperature of 42degrees, 50degrees or whatever other base temperature of interest) then all methods are fairly comparable. However, differences do occur and these are accentuated in an exceptionally long period of cool spring temperatures, such as we experienced this year. Let me briefly explain: 1. Averaging Method: Easy to calculate Degree Days(DD) = Average daily temp. - Base Temp. = (max. + min.) / 2 - Base temp. If answer is negative, assume 0. Example: Calculate DD Base 50 given 65 degrees max. and 40 degrees min. Avg. = (65 + 40) / 2 = 52.5 degrees DD base 50 = 52.5 - 50 = 2.5 degrees. But look what happens given a maximum of 60 degrees and a minimum of 35 degrees: Avg. = (60 + 35) / 2 = 47.5 DD base 50 = 47.5 - 50 = -2.5 = 0 degrees. The maximum temperature was higher than the base of 50° , but no degree days were accumulated. A grower called about the first of June reporting only about 40% of the DD50 that we have recorded. -

Having Regard to the Opinion of the European Chapter 1 of the Annex Binding Within Five Years of Parliament1 ; the Date of Entry Into Force of This Directive
878 Official Journal of the European Communities 29.10.71 Official Journal of the European Communities No L 243/29 COUNCIL DIRECTIVE of 18 October 1971 on the approximation of the laws of the Member States relating to units of measurement (71/354/EEC ) THE COUNCIL OF THE EUROPEAN COMMUNITIES, particular their names, symbols and use are not identical in the Member countries ; Having regard to the Treaty establishing the European Economic Community, and in particular HAS ADOPTED THIS DIRECTIVE : Article 100 thereof; Article 1 Having regard to the proposal from the Commission ; 1 . Member States shall make the provisions of Having regard to the Opinion of the European Chapter 1 of the Annex binding within five years of Parliament1 ; the date of entry into force of this Directive. 2 . Member States shall, with effect from 31 Having regard to the Opinion of the Economic and December 1977 at the latest, prohibit the use of the Social Committee2; units of measurement listed in Chapter III of the Annex. Whereas - the laws which regulate the use of units of measurement in the Member States differ from one 3 . The units of measurement temporarily" retained Member State to another and therefore hinder trade ; in accordance with the provisions of Chapter II or whereas application of the rules relating to measuring Chapter III of the Annex may not be brought into instruments is closely linked to the use of units of compulsory use by the Member States where they ' are measurement in the metrological system ; whereas, in not authorised at the date when this Directive enters into force . -

Angles ANGLE Topics • Coterminal Angles • Defintion of an Angle
Angles ANGLE Topics • Coterminal Angles • Defintion of an angle • Decimal degrees to degrees, minutes, seconds by hand using the TI-82 or TI-83 Plus • Degrees, seconds, minutes changed to decimal degree by hand using the TI-82 or TI-83 Plus • Standard position of an angle • Positive and Negative angles ___________________________________________________________________________ Definition: Angle An angle is created when a half-ray (the initial side of the angle) is drawn out of a single point (the vertex of the angle) and the ray is rotated around the point to another location (becoming the terminal side of the angle). An angle is created when a half-ray (initial side of angle) A: vertex point of angle is drawn out of a single point (vertex) AB: Initial side of angle. and the ray is rotated around the point to AC: Terminal side of angle another location (becoming the terminal side of the angle). Hence angle A is created (also called angle BAC) STANDARD POSITION An angle is in "standard position" when the vertex is at the origin and the initial side of the angle is along the positive x-axis. Recall: polynomials in algebra have a standard form (all the terms have to be listed with the term having the highest exponent first). In trigonometry, there is a standard position for angles. In this way, we are all talking about the same thing and are not trying to guess if your math solution and my math solution are the same. Not standard position. Not standard position. This IS standard position. Initial side not along Initial side along negative Initial side IS along the positive x-axis. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Applications of Geometry and Trigonometry
P1: FXS/ABE P2: FXS 9780521740517c14.xml CUAU031-EVANS September 6, 2008 13:36 CHAPTER 14 MODULE 2 Applications of geometry and trigonometry How do we apply trigonometric techniques to problems involving angles of depression and elevation? How do we apply trigonometric techniques to problems involving bearings? How do we apply trigonometric techniques to three-dimensional problems? How do we draw and interpret contour maps? How do we draw and interpret scale drawings? 14.1 Angles of elevation and depression, bearings, and triangulation Angles of elevation and depression The angle of elevation is the angle The angle of depression is the angle between the horizontal and a between the horizontal and a direction below direction above the horizontal. the horizontal. eye level line of sight angle of depression line of sight eye level cliff angle of elevation SAMPLEboat Cambridge University Press • Uncorrected Sample pages • 978-0-521-61328-6 • 2008 © Jones, Evans, Lipson TI-Nspire & Casio ClassPad material in collaboration with Brown410 and McMenamin P1: FXS/ABE P2: FXS 9780521740517c14.xml CUAU031-EVANS September 6, 2008 13:36 Chapter 14 — Applications of geometry and trigonometry 411 Bearings The three-figure bearing (or compass bearing) is the direction measured clockwise from north. The bearing of A from O is 030◦. The bearing of C from O is 210◦. The bearing of B from O is 120◦. The bearing of D from O is 330◦. N D N A 30° 330° E 120° E W O 210° W O B C S S Example 1 Angle of depression The pilot of a helicopter flying at 400 m observes a small boat at an angle of depression of 1.2◦. -

1. What Is the Radian Measure of an Angle Whose Degree Measure Is 72◦ 180◦ 72◦ = Πradians Xradians Solving for X 72Π X = Radians 180 2Π X = Radians 5 Answer: B
1. What is the radian measure of an angle whose degree measure is 72◦ 180◦ 72◦ = πradians xradians Solving for x 72π x = radians 180 2π x = radians 5 Answer: B 2. What is the length of AC? Using Pythagorean Theorem: 162 + b2 = 202 256 + b2 = 400 b2 = 144 b = 12 Answer: E 3. one solution to z2 + 64 = 0 is z2 + 64 = 0 z2 = −64 p p z2 = −64 Since there is a negative under the radical, we get imaginary roots. Thus z = −8i or z = +8i Answer: A 4. Simplifying: p 36x10y12 − 36y12 = p36y12 (x10 − 1) p = 36y12p(x10 − 1) p = 6y6 x10 − 1 Answer: C 1 5. Simplifying: 1 1 1 1 1 −3 9 6 3 −3 9 6 27a b c = 27 3 a 3 b 3 c 3 = 3a−1b3c2 3b3c2 = a Answer: C 6. Solving for x: p 8 + x + 14 = 12 p x + 14 = 12 − 8 p x + 14 = 4 p 2 x + 14 = 42 x + 14 = 16 x = 16 − 14 x = 2 Answer: C 7. Simplify x2 − 9 (x + 1)2 2x − 6 × ÷ x2 − 1 (2x + 3)(x + 3) 1 − x Rewrite as a multiplication. x2 − 9 (x + 1)2 1 − x × x2 − 1 (2x + 3)(x + 3) 2x − 6 Simplify each polynomial (x − 3)(x + 3) (x + 1)(x + 1) 1 − x × (x − 1)(x + 1) (2x + 3)(x + 3) 2(x − 3) Canceling like terms (x + 1) − 2(2x + 3) Answer: C 2 8. Simplifying: 1 11 x−5 11 + 2 2 + 2 x−5 (x−5) = (x−5) (x−5) x + 1 x + 1 x−5+11 2 = (x−5) x + 1 x+6 2 = (x−5) x + 1 x + 6 = (x − 5)2(x + 1) Answer: B 9. -

4.1 – Radian and Degree Measure
4.1 { Radian and Degree Measure Accelerated Pre-Calculus Mr. Niedert Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 1 / 27 2 Radian Measure 3 Degree Measure 4 Applications 4.1 { Radian and Degree Measure 1 Angles Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 2 / 27 3 Degree Measure 4 Applications 4.1 { Radian and Degree Measure 1 Angles 2 Radian Measure Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 2 / 27 4 Applications 4.1 { Radian and Degree Measure 1 Angles 2 Radian Measure 3 Degree Measure Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 2 / 27 4.1 { Radian and Degree Measure 1 Angles 2 Radian Measure 3 Degree Measure 4 Applications Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 2 / 27 The starting position of the ray is the initial side of the angle. The position after the rotation is the terminal side of the angle. The vertex of the angle is the endpoint of the ray. Angles An angle is determine by rotating a ray about it endpoint. Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 3 / 27 The position after the rotation is the terminal side of the angle. The vertex of the angle is the endpoint of the ray. Angles An angle is determine by rotating a ray about it endpoint. The starting position of the ray is the initial side of the angle. Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 3 / 27 The vertex of the angle is the endpoint of the ray. -
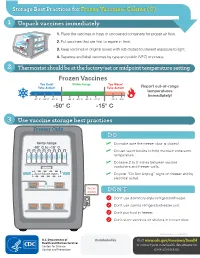
Storage Best Practices for Frozen Vaccines-Celsius
Storage Best Practices for Frozen Vaccines–Celsius (C) 1 Unpack vaccines immediately 1. Place the vaccines in trays or uncovered containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -57° C -54° C -51° C -46° C -43° C -40° C -37° C -12° C -9°C -50° C -15° C 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -50° C to -15° C ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision %FDFNCFS 20 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. -

Relationships of the SI Derived Units with Special Names and Symbols and the SI Base Units
Relationships of the SI derived units with special names and symbols and the SI base units Derived units SI BASE UNITS without special SI DERIVED UNITS WITH SPECIAL NAMES AND SYMBOLS names Solid lines indicate multiplication, broken lines indicate division kilogram kg newton (kg·m/s2) pascal (N/m2) gray (J/kg) sievert (J/kg) 3 N Pa Gy Sv MASS m FORCE PRESSURE, ABSORBED DOSE VOLUME STRESS DOSE EQUIVALENT meter m 2 m joule (N·m) watt (J/s) becquerel (1/s) hertz (1/s) LENGTH J W Bq Hz AREA ENERGY, WORK, POWER, ACTIVITY FREQUENCY second QUANTITY OF HEAT HEAT FLOW RATE (OF A RADIONUCLIDE) s m/s TIME VELOCITY katal (mol/s) weber (V·s) henry (Wb/A) tesla (Wb/m2) kat Wb H T 2 mole m/s CATALYTIC MAGNETIC INDUCTANCE MAGNETIC mol ACTIVITY FLUX FLUX DENSITY ACCELERATION AMOUNT OF SUBSTANCE coulomb (A·s) volt (W/A) C V ampere A ELECTRIC POTENTIAL, CHARGE ELECTROMOTIVE ELECTRIC CURRENT FORCE degree (K) farad (C/V) ohm (V/A) siemens (1/W) kelvin Celsius °C F W S K CELSIUS CAPACITANCE RESISTANCE CONDUCTANCE THERMODYNAMIC TEMPERATURE TEMPERATURE t/°C = T /K – 273.15 candela 2 steradian radian cd lux (lm/m ) lumen (cd·sr) 2 2 (m/m = 1) lx lm sr (m /m = 1) rad LUMINOUS INTENSITY ILLUMINANCE LUMINOUS SOLID ANGLE PLANE ANGLE FLUX The diagram above shows graphically how the 22 SI derived units with special names and symbols are related to the seven SI base units. In the first column, the symbols of the SI base units are shown in rectangles, with the name of the unit shown toward the upper left of the rectangle and the name of the associated base quantity shown in italic type below the rectangle. -
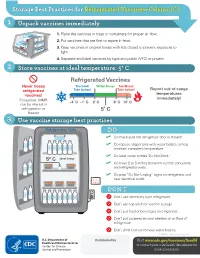
Storage Best Practices for Refrigerated Vaccines–Celsius (C)
Storage Best Practices for Refrigerated Vaccines–Celsius (C) 1 Unpack vaccines immediately 1. Place the vaccines in trays or containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Store vaccines at ideal temperature: 5° C Refrigerated Vaccines Never freeze Too Cold! Within Range Too Warm! refrigerated Take Action! Take Action! Report out-of-range vaccines! temperatures Exception: MMR immediately! -4˚ C -1˚ C 2˚ C 8˚ C 10˚ C can be stored in refrigerator or 5˚ C freezer 3 Use vaccine storage best practices Refrigerator Only DO ✓ Do make sure the refrigerator door is closed! ✓ Do replace crisper bins with water bottles to help maintain consistent temperature. ✓ Do label water bottles "Do Not Drink." 5° C ideal temp ✓ Do leave 2 to 3 inches between vaccine containers and refrigerator walls. ✓ Do post “Do Not Unplug” signs on refrigerator and do not near electrical outlet. unplug DON’T ; Don’t use dormitory-style refrigerator. ; Don’t use top shelf for vaccine storage. ; Don’t put food or beverages in refrigerator. ; Don’t put vaccines on door shelves or on floor of refrigerator. Don’t drink from or remove water bottles. ; CS243541-C Revision February 2018 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Can you find at least 8 things that are wrong with vaccine storage in this refrigerator? 5° C ideal temp 2 When unpacking vaccines, why is it important to put the first-to-expire in the front? A. -

The International System of Units (SI)
NAT'L INST. OF STAND & TECH NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 330 2001 Edition The International System of Units (SI) 4. Barry N. Taylor, Editor r A o o L57 330 2oOI rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -

Innovative Concepts and Technology for Railroad-Highway Grade
NOTICE This document is disseminated under the sponsorship of the Department of Transportation in the interest of information exchange. The United States Govern ment assumes no liability for its contents or use thereof. NOTICE The United States Government does not endorse pro ducts or manufacturers. Trade or manufacturers' names appear herein solely because they are con sidered essential to the object of this report. T ~chnical Repart Docum~ntotion Poge 1. RopOr! No. 12. G.y.,"~.", Acce",," No. 3. Reciplenl's Colalog No ;- FRA/ORD - 77/37 .II ~b -1-'1 ~~ ') - '?> I 4. Title and Subtitle 5. Reporr Dale INKOVATIVE CONCEPTS AND TECHNOLOGY FOR September 1977 I RAILROAD -HIGH1\'AY GRADE CROSSING MOTORIST 6. Pe,Iorrnlng Organ, 101 on Code' t WARNING SYSTEl>lS Vol. II: The Generation and ~ Analysis of Alternative Concepts 8. Pe,formlng Orgonl10tlon Reperr No. 7. Au1f-or's) : D.O. Peterson and D.S. Boyer DOT-TSC-FRA-76-l9.II 9. Per'OfrT\lng Orgar1l10tio... Name and Address 10. Wor ... Unl' No. (TRAIS) Tracor-Jitco, Inc. RR602/R7337 * I 11. Contract Or Granl No. i 1776 East Jefferson Street : DOT-TSC-842-2 Rockville MD 20852 , 13. l)'pe of Report and Per·cei Covered 12. Spon,oring Agency Name and Addren I U.S. Department of Transportation , Final Report : Federal Railroad Administration June 1974 -ylarch 1976 Office of Research and Development 14. SpanlGring A90ner Code Washington DC 20590 15. Supplemenlary Notes : U.S. Department of Transportation !*Under contract to: Transportation Systems Center I Kendall Square Cambridge t-IA 02142 I 16. Abstroct I This report describes the results of a study directed toward the I generation, analysis and evaluation of innovative conceptual and technical approahces to train-activated motorist warning systems for i use at railroad-highway grade crossings.