The Effects of Climate Change on Migratory Waterbirds Within the African-Eurasian Flyway
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wetland Birds in the Recent Fossil Record of Britain and Northwest Europe John R
Wetland birds in the recent fossil record of Britain and northwest Europe John R. Stewart 18. Dalmatian Pelican Pelecanus crispus, Deep Bay, Mai Po, Hong Kong, February 1995. Geological evidence suggests that Dalmatian Pelicans bred in Britain, and in other western European countries (including The Netherlands and Denmark), prior to and during the Iron Age. Ray Tipper. ABSTRACT Wetland habitats in Britain and other parts of western Europe have been severely depleted during the latter part of the Holocene owing principally to drainage and land reclamation. Changes in the distribution of a number of wetland bird species can be gauged from archaeological and geological site records of larger birds, whose remains are generally better preserved. Key species are discussed here, including a heron Nycticorax fenensis and a crane Grus primigenia, two extinct species named on possibly uncertain fossil evidence. We can let our minds wander back to the misty realms of fifteen hundred years ago, to a wonderful Britain which was alive with bird song from coast to coast, which sheltered wolves, bears and boars in its dark woodlands, cranes in its marshes, bustards on its heaths and beavers by its streams, and we can visualize the great pink pelican sweeping on its huge pinions over the reedy waterways which then penetrated by secret paths into the very heart of what is now Somerset. (Whitlock, 1953) © British Birds 97 • January 2004 • 33-43 33 Wetland birds in the recent fossil record f all the major habitats in northwest species, including Mute Swan Cygnus olor and Europe, wetlands may have been the Common Crane, may have become physically Omost severely depleted during the smaller owing to habitat impoverishment. -

Status and Protection of Globally Threatened Species in the Caucasus
STATUS AND PROTECTION OF GLOBALLY THREATENED SPECIES IN THE CAUCASUS CEPF Biodiversity Investments in the Caucasus Hotspot 2004-2009 Edited by Nugzar Zazanashvili and David Mallon Tbilisi 2009 The contents of this book do not necessarily reflect the views or policies of CEPF, WWF, or their sponsoring organizations. Neither the CEPF, WWF nor any other entities thereof, assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, product or process disclosed in this book. Citation: Zazanashvili, N. and Mallon, D. (Editors) 2009. Status and Protection of Globally Threatened Species in the Caucasus. Tbilisi: CEPF, WWF. Contour Ltd., 232 pp. ISBN 978-9941-0-2203-6 Design and printing Contour Ltd. 8, Kargareteli st., 0164 Tbilisi, Georgia December 2009 The Critical Ecosystem Partnership Fund (CEPF) is a joint initiative of l’Agence Française de Développement, Conservation International, the Global Environment Facility, the Government of Japan, the MacArthur Foundation and the World Bank. This book shows the effort of the Caucasus NGOs, experts, scientific institutions and governmental agencies for conserving globally threatened species in the Caucasus: CEPF investments in the region made it possible for the first time to carry out simultaneous assessments of species’ populations at national and regional scales, setting up strategies and developing action plans for their survival, as well as implementation of some urgent conservation measures. Contents Foreword 7 Acknowledgments 8 Introduction CEPF Investment in the Caucasus Hotspot A. W. Tordoff, N. Zazanashvili, M. Bitsadze, K. Manvelyan, E. Askerov, V. Krever, S. Kalem, B. Avcioglu, S. Galstyan and R. Mnatsekanov 9 The Caucasus Hotspot N. -

The Good, the Bad and the Ugly of COVID-19 Lockdown Effects on Wildlife Conservation Insights from the First European Locked Do
Biological Conservation 249 (2020) 108728 Contents lists available at ScienceDirect Biological Conservation journal homepage: www.elsevier.com/locate/biocon Perspective The good, the bad and the ugly of COVID-19 lockdown effects on wildlife conservation: Insights from the first European locked down country T ⁎ Raoul Manentia, , Emiliano Morib, Viola Di Canioa, Silvia Mercurioa, Marco Piconec, Mario Caffid, Mattia Brambillae,f, Gentile Francesco Ficetolaa,g, Diego Rubolinia a Dipartimento di Scienze e Politiche Ambientali, Università degli Studi di Milano, via Celoria 26, I-20133 Milano, Italy b Consiglio Nazionale delle Ricerche, Istituto di Ricerca sugli Ecosistemi Terrestri, Via Madonna del Piano 10, 50019 Sesto Fiorentino, Italy c Dipartimento di Scienze Ambientali, Informatica e Statistica, Università Ca' Foscari di Venezia, Via Torino 55, I-30172 Venezia, Italy d Osservatorio Ornitologico Pianura Bresciana ‘Padernello’, via Cavour 1, I-25022 Borgo San Giacomo, BS, Italy e Fondazione Lombardia per l'Ambiente, Settore biodiversità e aree protette, Largo 10 luglio 1976 1, I-20822 Seveso, MB, Italy f Museo delle Scienze, Sezione di Zoologia dei Vertebrati, Corso del Lavoro e della Scienza 3, I-38122 Trento, Italy g Univ. Grenoble Alpes, CNRS, Univ. Savoie Mont Blanc, LECA, Laboratoire d'Ecologie Alpine, Rue de la Piscine 2233, F-38000 Grenoble, France ARTICLE INFO ABSTRACT Keywords: The COVID-19 pandemic zoonosis has determined extensive lockdowns worldwide that provide an un- Coronavirus precedented opportunity to understand how large-scale shifts of human activities can impact wildlife. We ad- Conservation dressed the impacts of the COVID-19 lockdown on wildlife in Italy, the first European country that performed a Crisis countrywide lockdown, and identified potentially beneficial and negative consequences for wildlife conservation Pandemic and management. -
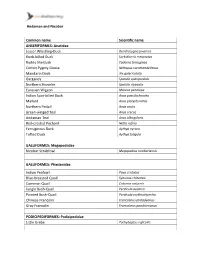
Andaman and Nicobar Common Name Scientific Name
Andaman and Nicobar Common name Scientific name ANSERIFORMES: Anatidae Lesser Whistling-Duck Dendrocygna javanica Knob-billed Duck Sarkidiornis melanotos Ruddy Shelduck Tadorna ferruginea Cotton Pygmy-Goose Nettapus coromandelianus Mandarin Duck Aix galericulata Garganey Spatula querquedula Northern Shoveler Spatula clypeata Eurasian Wigeon Mareca penelope Indian Spot-billed Duck Anas poecilorhyncha Mallard Anas platyrhynchos Northern Pintail Anas acuta Green-winged Teal Anas crecca Andaman Teal Anas albogularis Red-crested Pochard Netta rufina Ferruginous Duck Aythya nyroca Tufted Duck Aythya fuligula GALLIFORMES: Megapodiidae Nicobar Scrubfowl Megapodius nicobariensis GALLIFORMES: Phasianidae Indian Peafowl Pavo cristatus Blue-breasted Quail Synoicus chinensis Common Quail Coturnix coturnix Jungle Bush-Quail Perdicula asiatica Painted Bush-Quail Perdicula erythrorhyncha Chinese Francolin Francolinus pintadeanus Gray Francolin Francolinus pondicerianus PODICIPEDIFORMES: Podicipedidae Little Grebe Tachybaptus ruficollis Andaman and Nicobar COLUMBIFORMES: Columbidae Rock Pigeon Columba livia Andaman Wood-Pigeon Columba palumboides Eurasian Collared-Dove Streptopelia decaocto Red Collared-Dove Streptopelia tranquebarica Spotted Dove Streptopelia chinensis Laughing Dove Streptopelia senegalensis Andaman Cuckoo-Dove Macropygia rufipennis Asian Emerald Dove Chalcophaps indica Nicobar Pigeon Caloenas nicobarica Andaman Green-Pigeon Treron chloropterus Green Imperial-Pigeon Ducula aenea Nicobar Imperial-Pigeon Ducula nicobarica Pied Imperial-Pigeon -

Dalmatian Pelican Identification Manual Companion Document to the “Dalmatian Pelican Monitoring Manual”
Pelican Way of LIFE (LIFE18 NAT/NL/716) “Conservation of the Dalmatian Pelican along the Black Sea - Mediterranean Flyway” Dalmatian Pelican Identification Manual Companion document to the “Dalmatian Pelican Monitoring Manual” December 2020 Authored by: Commissioned by: Giorgos Catsadorakis and Olga Alexandrou, Society for the Protection of Prespa Rewilding Europe Dalmatian Pelican Identification Manual - Companion document to the “Dalmatian Pelican Monitoring Manual” December 2020 Authors: Giorgos Catsadorakis1 and Olga Alexandrou2 1,2Society for the Protection of Prespa, Agios Germanos, GR-53150, Prespa, Greece, [email protected] , [email protected] © Rewilding Europe Executed under the framework of “Conservation of the Dalmatian Pelican along the Black Sea - Mediterranean Flyway” (Pelican Way of LIFE; LIFE18 NAT/NL/716; https://life-pelicans.com/) project, financed by the LIFE programme of the European Union and Arcadia – a charitable fund of Lisbet Rausing and Peter Baldwin. Suggested citation: Society for the Protection of Prespa, 2020. Dalmatian pelican identification manual - Companion document to the “Dalmatian pelican monitoring manual”. Rewilding Europe. Produced within the framework of Pelican Way of LIFE project (LIFE18 NAT/NL/716). Keywords: Dalmatian pelican, identification, ageing, plumage, moulting patterns, sexing. Photo credits: Society for the Protection of Prespa, unless stated otherwise. Introduction Visual identification of the Dalmatian pelican seems like a straightforward task, yet in sites used by both species (the Dalmatian pelican and the great white pelican) identification can be tricky, especially when the observer is located some distance away from the birds. Hardly any useful material has been published on the sequence of moults and plumages of the Dalmatian pelican, and this gap often creates confusion and uncertainty about the accuracy of data in regards to ageing of Dalmatian pelicans, as well as frequently undermining confidence in census numbers. -

South Africa: Magoebaskloof and Kruger National Park Custom Tour Trip Report
SOUTH AFRICA: MAGOEBASKLOOF AND KRUGER NATIONAL PARK CUSTOM TOUR TRIP REPORT 24 February – 2 March 2019 By Jason Boyce This Verreaux’s Eagle-Owl showed nicely one late afternoon, puffing up his throat and neck when calling www.birdingecotours.com [email protected] 2 | TRIP REPORT South Africa: Magoebaskloof and Kruger National Park February 2019 Overview It’s common knowledge that South Africa has very much to offer as a birding destination, and the memory of this trip echoes those sentiments. With an itinerary set in one of South Africa’s premier birding provinces, the Limpopo Province, we were getting ready for a birding extravaganza. The forests of Magoebaskloof would be our first stop, spending a day and a half in the area and targeting forest special after forest special as well as tricky range-restricted species such as Short-clawed Lark and Gurney’s Sugarbird. Afterwards we would descend the eastern escarpment and head into Kruger National Park, where we would make our way to the northern sections. These included Punda Maria, Pafuri, and the Makuleke Concession – a mouthwatering birding itinerary that was sure to deliver. A pair of Woodland Kingfishers in the fever tree forest along the Limpopo River Detailed Report Day 1, 24th February 2019 – Transfer to Magoebaskloof We set out from Johannesburg after breakfast on a clear Sunday morning. The drive to Polokwane took us just over three hours. A number of birds along the way started our trip list; these included Hadada Ibis, Yellow-billed Kite, Southern Black Flycatcher, Village Weaver, and a few brilliant European Bee-eaters. -

Practical Guide for Breeding Ecology of Kentish Plover
Practical guide for investigating breeding ecology of Kentish plover Charadrius alexandrinus Tamás Székely, András Kosztolányi & Clemens Küpper Department of Biology & Biochemistry, University of Bath, Bath BA2 7AY, UK [email protected] [email protected] [email protected] Version 3, 1 April 2008 Photos by T. Székely, A. Kosztolányi & C. Küpper Rationale The Kentish/snowy plover Charadrius alexandrinus is a small cosmopolitan shorebird (body mass about 40-44 g). In the last few years we have developed a suite of methods to investigate its behaviour and ecology in the field. We thought this practical guide may be useful for students and researchers with an interest in small plovers. Some aspects of these methods may be relevant for other shorebirds and ground-nesting birds in general. Our fundamental motivation in writing this guide is to show that the Kentish plover is an easy species to work with, if one is willing to pay attention to a few potential pitfalls. We hope that this guide will elicit further research. Please contact us if you have questions and comments, and let us know of any errors. Note that Kentish plovers have been studied in several countries and by a good range of researchers, and we don't claim that our methods work best. Many Kentish plover populations are now declining. You need to be sensible about fieldwork, and carefully evaluate the costs and benefits of using a particular method. The last thing you want is to put an extra burden on plover populations - they have a hard time anyway to cope with predators, floods and threats humans are imposing upon them. -

Adoption of Amendments to the Aewa Annexes
AGREEMENT ON THE CONSERVATION OF AFRICAN-EURASIAN MIGRATORY WATERBIRDS 6th SESSION OF THE MEETING OF THE PARTIES 9-14 November 2015, Bonn, Germany “Making flyway conservation happen” RESOLUTION 6.1 ADOPTION OF AMENDMENTS TO THE AEWA ANNEXES Recalling Article X of the Agreement concerning the procedures for amendments to the Agreement and its annexes, Further recalling Resolution 4.11 which, inter alia, requested the Technical Committee to review taxonomic classifications of birds and suggest the most appropriate classification for the purposes of the Agreement, including application to Annex 2, Recognising the work of the Technical Committee and the Secretariat over the past two triennia to address this request and their involvement and input into the work of the CMS Scientific Council Intersessional Working Group on Bird Taxonomy and Nomenclature from 2009 to 2011 and the Ad Hoc Meeting on Harmonisation of Bird Taxonomy which was convened by the Chair of the CMS Scientific Council in Formia, Italy on 8 October 2013, Noting the CMS Resolution 11.19 on the Taxonomy and Nomenclature of Birds Listed on the CMS Appendices adopted by COP11 (4-9 November 2014, Quito, Ecuador), Taking into account the recommendation of the Technical Committee, provided at its 12th Meeting on 3-6 March 2015 in Bonn, Germany, on the most appropriate classification for the purposes of the Agreement, Taking into account the findings of the sixth edition of the Report on the Conservation Status of Migratory Waterbirds in the Agreement Area (document AEWA/MOP 6.14), -

Bird Checklists of the World Country Or Region: Ghana
Avibase Page 1of 24 Col Location Date Start time Duration Distance Avibase - Bird Checklists of the World 1 Country or region: Ghana 2 Number of species: 773 3 Number of endemics: 0 4 Number of breeding endemics: 0 5 Number of globally threatened species: 26 6 Number of extinct species: 0 7 Number of introduced species: 1 8 Date last reviewed: 2019-11-10 9 10 Recommended citation: Lepage, D. 2021. Checklist of the birds of Ghana. Avibase, the world bird database. Retrieved from .https://avibase.bsc-eoc.org/checklist.jsp?lang=EN®ion=gh [26/09/2021]. Make your observations count! Submit your data to ebird. -

Review of the Status of Introduced Non-Native Waterbird Species in the Area of the African-Eurasian Waterbird Agreement: 2007 Update
Secretariat provided by the Workshop 3 United Nations Environment Programme (UNEP) Doc TC 8.25 21 February 2008 8th MEETING OF THE TECHNICAL COMMITTEE 03 - 05 March 2008, Bonn, Germany ___________________________________________________________________________ Review of the Status of Introduced Non-Native Waterbird Species in the Area of the African-Eurasian Waterbird Agreement: 2007 Update Authors A.N. Banks, L.J. Wright, I.M.D. Maclean, C. Hann & M.M. Rehfisch February 2008 Report of work carried out by the British Trust for Ornithology under contract to AEWA Secretariat © British Trust for Ornithology British Trust for Ornithology, The Nunnery, Thetford, Norfolk IP24 2PU Registered Charity No. 216652 CONTENTS Page No. List of Tables...........................................................................................................................................5 List of Figures.........................................................................................................................................7 List of Appendices ..................................................................................................................................9 EXECUTIVE SUMMARY..................................................................................................................11 RECOMMENDATIONS .....................................................................................................................13 1. INTRODUCTION.................................................................................................................15 -

Iucn Red Data List Information on Species Listed On, and Covered by Cms Appendices
UNEP/CMS/ScC-SC4/Doc.8/Rev.1/Annex 1 ANNEX 1 IUCN RED DATA LIST INFORMATION ON SPECIES LISTED ON, AND COVERED BY CMS APPENDICES Content General Information ................................................................................................................................................................................................................................ 2 Species in Appendix I ............................................................................................................................................................................................................................... 3 Mammalia ............................................................................................................................................................................................................................................ 4 Aves ...................................................................................................................................................................................................................................................... 7 Reptilia ............................................................................................................................................................................................................................................... 12 Pisces ................................................................................................................................................................................................................................................. -

References.Qxd 12/14/2004 10:35 AM Page 771
Ducks_References.qxd 12/14/2004 10:35 AM Page 771 References Aarvak, T. and Øien, I.J. 1994. Dverggås Anser Adams, J.S. 1971. Black Swan at Lake Ellesmere. erythropus—en truet art i Norge. Vår Fuglefauna 17: 70–80. Wildl. Rev. 3: 23–25. Aarvak, T. and Øien, I.J. 2003. Moult and autumn Adams, P.A., Robertson, G.J. and Jones, I.L. 2000. migration of non-breeding Fennoscandian Lesser White- Time-activity budgets of Harlequin Ducks molting in fronted Geese Anser erythropus mapped by satellite the Gannet Islands, Labrador. Condor 102: 703–08. telemetry. Bird Conservation International 13: 213–226. Adrian, W.L., Spraker, T.R. and Davies, R.B. 1978. Aarvak, T., Øien, I.J. and Nagy, S. 1996. The Lesser Epornitics of aspergillosis in Mallards Anas platyrhynchos White-fronted Goose monitoring programme,Ann. Rept. in north central Colorado. J. Wildl. Dis. 14: 212–17. 1996, NOF Rappportserie, No. 7. Norwegian Ornitho- AEWA 2000. Report on the conservation status of logical Society, Klaebu. migratory waterbirds in the agreement area. Technical Series Aarvak, T., Øien, I.J., Syroechkovski Jr., E.E. and No. 1.Wetlands International,Wageningen, Netherlands. Kostadinova, I. 1997. The Lesser White-fronted Goose Afton, A.D. 1983. Male and female strategies for Monitoring Programme.Annual Report 1997. Klæbu, reproduction in Lesser Scaup. Unpubl. Ph.D. thesis. Norwegian Ornithological Society. NOF Raportserie, Univ. North Dakota, Grand Forks, US. Report no. 5-1997. Afton, A.D. 1984. Influence of age and time on Abbott, C.C. 1861. Notes on the birds of the Falkland reproductive performance of female Lesser Scaup.