Hemigrapsus Oregonensis, Cancer Gracilis, and P
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SCAMIT Newsletter Vol. 11 No. 12 1993 April
f^fO^'M Southern California Association of Marine Invertebrate Taxonomists 3720 Stephen White Drive San Pedro, California 90731 April, 1993 Vol. 11, Nb.12 NEXT MEETING: Master Species List GUEST SPEAKER: None DATE: May 10,1993 9:30 am - 3:00 pm LOCATION: Cabrillo Marine Museum San Pedro, CA MAY 10 MEETING The meeting will be devoted to working on the master species list. We will be resolving the final version of the list containing the four major dischargers and discussing the addition of the minor dischargers. FUNDS FOR THIS PUBLICATION PROVIDED IN PART BY THE ARCO FOUNDATION, CHEVRON USA, AND TEXACO INC. SCAM1T Newsletter is not deemed to be a valid publication for formal taxonomic purposes. MINUTES FROM MEETING ON APRIL 12 Larry Lovell is looking for suggestions for have SCAMIT members volunteer to assist possible speakers and subjects (especially ontripsasknowledgeableguides. Jodi is also non-polychaete taxa) for the next year. He laying plans for a Crustacean Biodiversity would appreciate any input you might have. workshop. He will contact international You can write him at: experts on as many families as possible to get Larry Lovell estimates onnumber of known and remaining 1036 Buena Vista species to be described. Vista, CA 92083 Jodi began by discussing Decapod higher taxonomy. Based on Spears et al. (1992) Larry announced again that for the 1994 Brachyura and Anomura are clearly Annual Meeting of the Southern California differentiated by sperm. Dromidia Academy of Sciences SCAMIT might be able (Dromiacea) and Litkodids (Alaskan King to have a taxonomic symposium. Also crab) have been confirmed as anomurans by discussed was the possibility of SCAMTT recent research. -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber ......................................................................... -

Physiological Adaptations and Feeding Mechanisms of the Invasive Purple Varnish Clam, Nuttallia Obscurata
Western Washington University Western CEDAR WWU Graduate School Collection WWU Graduate and Undergraduate Scholarship 2013 Physiological adaptations and feeding mechanisms of the invasive purple varnish clam, Nuttallia obscurata Leesa E. Sorber Western Washington University Follow this and additional works at: https://cedar.wwu.edu/wwuet Part of the Biology Commons Recommended Citation Sorber, Leesa E., "Physiological adaptations and feeding mechanisms of the invasive purple varnish clam, Nuttallia obscurata" (2013). WWU Graduate School Collection. 281. https://cedar.wwu.edu/wwuet/281 This Masters Thesis is brought to you for free and open access by the WWU Graduate and Undergraduate Scholarship at Western CEDAR. It has been accepted for inclusion in WWU Graduate School Collection by an authorized administrator of Western CEDAR. For more information, please contact [email protected]. PHYSIOLOGICAL ADAPTATIONS AND FEEDING MECHANISMS OF THE INVASIVE PURPLE VARNISH CLAM, NUTTALLIA OBSCURATA by Leesa E. Sorber Accepted in Partial Completion of the Requirements for the Degree Master of Science Kathleen L. Kitto, Dean of the Graduate School ADVISORY COMMITTEE Chair, Dr. Deborah Donovan Dr. Benjamin Miner Dr. Jose Serrano-Moreno MASTER’S THESIS In presenting this thesis in partial fulfillment of the requirements for a master’s degree at Western Washington University, I grant Western Washington University the non-exclusive royalty-free right to archive, reproduce, distribute, and display the thesis in any and all forms, including electronic format, via any digital library mechanisms maintained by WWU. I represent and warrant this is my original work, and does not infringe or violate any rights of others. I warrant that I have obtained written permission for the owner of any third party copyrighted material included in these files. -

On the Oceanic Transport of Crab Larval Stages*
Reprinted from the "Proceedings of Symposium on Crustacea"—Part I ON THE OCEANIC TRANSPORT OF CRAB LARVAL STAGES* JOHN S. GARTH Allan Hancock Foundation, University of Southern California, Los Angeles, California, U.S.A. ABSTRACT Evidence from the disciplines of oceanography, marine geology, crustacean physiology, life-history and faunistic studies is marshalled in an attempt to demonstrate the probable manner in which fragile-bodied and short-lived crab zoeae and megalopa have been able to span ocean barriers, following major ocean currents as highways and using remote islands as stepping-stones to continental shores. While the concept has been developed in connection with distribution studies of crabs of western America and its offshore islands in the eastern Pacific, it is believed applicable with suitable refinement to other continents and their outlying islands in other seas. INTRODUCTION IT is nearly 20 years since the writer postulated, in connection with studies on Galapagos Brachyura, that "the system of oceanic circulation observed in the Galapagos Islands, plus that known to exist in the greater. Pacific area, are together capable of accounting for the recognized distribution of brachyuran species within the archipelago on the basis of oceanic transportation of larval stages alone" (Garth, 1946, p. 617). In that study the California, Nino, Peru, and Equa torial Counter currents were designated, either singly or in combination, as the agencies responsible for the transporting of larval stages to the archipelago from the Baja California-Gulf of California region, the Bay of Panama, the South American west coast, and trans-Pacific islands. As a corollary the role of intervening islands as way-stations was recognized : "The fact that other Galapagos species are common to the intermediate outposts of Clarion and Socorro... -

Larval Growth
LARVAL GROWTH Edited by ADRIAN M.WENNER University of California, Santa Barbara OFFPRINT A.A.BALKEMA/ROTTERDAM/BOSTON DARRYL L.FELDER* / JOEL W.MARTIN** / JOSEPH W.GOY* * Department of Biology, University of Louisiana, Lafayette, USA ** Department of Biological Science, Florida State University, Tallahassee, USA PATTERNS IN EARLY POSTLARVAL DEVELOPMENT OF DECAPODS ABSTRACT Early postlarval stages may differ from larval and adult phases of the life cycle in such characteristics as body size, morphology, molting frequency, growth rate, nutrient require ments, behavior, and habitat. Primarily by way of recent studies, information on these quaUties in early postlarvae has begun to accrue, information which has not been previously summarized. The change in form (metamorphosis) that occurs between larval and postlarval life is pronounced in some decapod groups but subtle in others. However, in almost all the Deca- poda, some ontogenetic changes in locomotion, feeding, and habitat coincide with meta morphosis and early postlarval growth. The postmetamorphic (first postlarval) stage, here in termed the decapodid, is often a particularly modified transitional stage; terms such as glaucothoe, puerulus, and megalopa have been applied to it. The postlarval stages that fol low the decapodid successively approach more closely the adult form. Morphogenesis of skeletal and other superficial features is particularly apparent at each molt, but histogenesis and organogenesis in early postlarvae is appreciable within intermolt periods. Except for the development of primary and secondary sexual organs, postmetamorphic change in internal anatomy is most pronounced in the first several postlarval instars, with the degree of anatomical reorganization and development decreasing in each of the later juvenile molts. -

Phylogeny of the Genus Pinnixa White, 1846
DIRECTEUR DE LA PUBLICATION : Bruno David Président du Muséum national d’Histoire naturelle RÉDACTRICE EN CHEF / EDITOR-IN-CHIEF : Laure Desutter-Grandcolas ASSISTANTS DE RÉDACTION / ASSISTANT EDITORS : Anne Mabille ([email protected]) MISE EN PAGE / PAGE LAYOUT : Anne Mabille COMITÉ SCIENTIFIQUE / SCIENTIFIC BOARD : James Carpenter (AMNH, New York, États-Unis) Maria Marta Cigliano (Museo de La Plata, La Plata, Argentine) Henrik Enghoff (NHMD, Copenhague, Danemark) Rafael Marquez (CSIC, Madrid, Espagne) Peter Ng (University of Singapore) Norman I. Platnick (AMNH, New York, États-Unis) Jean-Yves Rasplus (INRA, Montferrier-sur-Lez, France) Jean-François Silvain (IRD, Gif-sur-Yvette, France) Wanda M. Weiner (Polish Academy of Sciences, Cracovie, Pologne) John Wenzel (The Ohio State University, Columbus, États-Unis) COUVERTURE / COVER : Morphological characters of the type species of some genera within subfamily Pinnixinae Števčić, 2005. Zoosystema est indexé dans / Zoosystema is indexed in: – Science Citation Index Expanded (SciSearch®) – ISI Alerting Services® – Current Contents® / Agriculture, Biology, and Environmental Sciences® – Scopus® Zoosystema est distribué en version électronique par / Zoosystema is distributed electronically by: – BioOne® (http://www.bioone.org) Les articles ainsi que les nouveautés nomenclaturales publiés dans Zoosystema sont référencés par / Articles and nomenclatural novelties published in Zoosystema are referenced by: – ZooBank® (http://zoobank.org) Zoosystema est une revue en flux continu publiée par les Publications scientifiques du Muséum, Paris / Zoosystema is a fast track journal published by the Museum Science Press, Paris Les Publications scientifiques du Muséum publient aussi / The Museum Science Press also publish: Adansonia, Geodiversitas, Anthropozoologica, European Journal of Taxonomy, Naturae, Cryptogamie sous-sections Algologie, Bryologie, Mycologie. Diffusion – Publications scientifiques Muséum national d’Histoire naturelle CP 41 – 57 rue Cuvier F-75231 Paris cedex 05 (France) Tél. -

King County Zooplankton Monitoring Annual Report 2017
King County Zooplankton Monitoring Annual Report 2017 31 August 2018 Dr. Julie E. Keister Box 357940 Seattle, WA 98195 (206) 543-7620 [email protected] Prepared by: Dr. Julie E. Keister, Amanda Winans, and BethElLee Herrmann King County Zooplankton Monitoring Annual Report 2017 Project Oversight and Report Preparation The zooplankton analyses reported herein were conducted in Dr. Julie E. Keister’s laboratory at the University of Washington, School of Oceanography. Dr. Keister designed the protocols for the field zooplankton sampling and laboratory analysis. Field sampling was conducted by the King County Department of Natural Resources and Parks, Water and Land Resources Division. Taxonomic analysis was conducted by Amanda Winans, BethElLee Herrmann, and Michelle McCartha at the University of Washington. This report was prepared by Winans and Herrmann, with oversight by Dr. Keister. Acknowledgments We would like to acknowledge the following individuals and organizations for their contributions to the successful 2017 sampling and analysis of the King County zooplankton monitoring in the Puget Sound: From King County, we thank Kimberle Stark, Wendy Eash-Loucks, the King County Environmental Laboratory field scientists, and the captain and crew of the R/V SoundGuardian. We would also like to thank our collaborators Moira Galbraith and Kelly Young from Fisheries and Oceans Canada Institute of Ocean Sciences for their expert guidance in species identification and Cheryl Morgan from Oregon State University for assistance in designing sampling and analysis protocols. King County Water and Land Resources Division provided funding for these analyses, with supplemental funding provided by Long Live the Kings for analysis of oblique tow (bongo net) samples as part of the Salish Sea Marine Survival Project. -
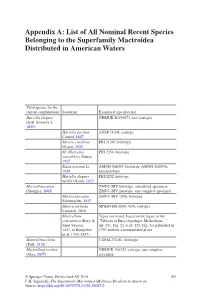
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -

Kachemak Bay Research Reserve: a Unit of the National Estuarine Research Reserve System
Kachemak Bay Ecological Characterization A Site Profile of the Kachemak Bay Research Reserve: A Unit of the National Estuarine Research Reserve System Compiled by Carmen Field and Coowe Walker Kachemak Bay Research Reserve Homer, Alaska Published by the Kachemak Bay Research Reserve Homer, Alaska 2003 Kachemak Bay Research Reserve Site Profile Contents Section Page Number About this document………………………………………………………………………………………………………… .4 Acknowledgements…………………………………………………………………………………………………………… 4 Introduction to the Reserve ……………………………………………………………………………………………..5 Physical Environment Climate…………………………………………………………………………………………………… 7 Ocean and Coasts…………………………………………………………………………………..11 Geomorphology and Soils……………………………………………………………………...17 Hydrology and Water Quality………………………………………………………………. 23 Marine Environment Introduction to Marine Environment……………………………………………………. 27 Intertidal Overview………………………………………………………………………………. 30 Tidal Salt Marshes………………………………………………………………………………….32 Mudflats and Beaches………………………………………………………………………… ….37 Sand, Gravel and Cobble Beaches………………………………………………………. .40 Rocky Intertidal……………………………………………………………………………………. 43 Eelgrass Beds………………………………………………………………………………………… 46 Subtidal Overview………………………………………………………………………………… 49 Midwater Communities…………………………………………………………………………. 51 Shell debris communities…………………………………………………………………….. 53 Subtidal soft bottom communities………………………………………………………. 54 Kelp Forests…………………………………………………….…………………………………….59 Terrestrial Environment…………………………………………………………………………………………………. 61 Human Dimension Overview………………………………………………………………………………………………. -

(Linnaeus) and Its Parasite Sacculina Carcini Thompson in Burma, with Notes on the Transport of Crabs to New Localities
ON THE OCCURRENCE OF CARCINUS MAENAS (LINNAEUS) AND ITS PARASITE SACCULINA CARCINI THOMPSON IN BURMA, WITH NOTES ON THE TRANSPORT OF CRABS TO NEW LOCALITIES by H. BOSGHMA Rijksmuseum van Natuurlijke Historie, Leiden From time to time crabs have been found in localities at great distances from their normal area of distribution, owing to their being transported by ships in ballast water tanks or on the hulls. Much attention has been paid to a report by Catta (1876), who examined the Crustacea taken from the hull of a ship that had made the voyage from India to Marseilles. Among these, four species of crabs of the family Grapsidae were represented, one of which, named by him Plagusia squamosa, being present in hundreds of specimens. Catta saw here the possibility of an extension of the area of distribution of the crab, but apparently the conditions of life were not favourable in the new locality, and the crab did not become established here. Stebbing (1893: 99) remarks that the correct name of the crab referred to above is Plagusia depressa (Fabricius) and adds that "The point of special interest, however, lies, as Catta explains, in showing the effects on distribution that may be produced by unconscious human agency." The data mentioned above were recorded again by Chilton (1911). According to Bennett (1964: 87) lists as given by Stebbing and Chilton of species transported on the hulls of ships are not lists of accidental acclimatisations, for it does not follow that the crabs could establish themselves in their new homes. Another case of unsuccessful transport of a crab to a new locality is the record of great numbers of Pilumnus spinifer H. -

An Overview of the Decapoda with Glossary and References
January 2011 Christina Ball Royal BC Museum An Overview of the Decapoda With Glossary and References The arthropods (meaning jointed leg) are a phylum that includes, among others, the insects, spiders, horseshoe crabs and crustaceans. A few of the traits that arthropods are characterized by are; their jointed legs, a hard exoskeleton made of chitin and growth by the process of ecdysis (molting). The Crustacea are a group nested within the Arthropoda which includes the shrimp, crabs, krill, barnacles, beach hoppers and many others. The members of this group present a wide range of morphology and life history, but they do have some unifying characteristics. They are the only group of arthropods that have two pairs of antenna. The decapods (meaning ten-legged) are a group within the Crustacea and are the topic of this key. The decapods are primarily characterized by a well developed carapace and ten pereopods (walking legs). The higher-level taxonomic groups within the Decapoda are the Dendrobranchiata, Anomura, Brachyura, Caridea, Astacidea, Axiidea, Gebiidea, Palinura and Stenopodidea. However, two of these groups, the Palinura (spiny lobsters) and the Stenopodidea (coral shrimps), do not occur in British Columbia and are not dealt with in this key. The remaining groups covered by this key include the crabs, hermit crabs, shrimp, prawns, lobsters, crayfish, mud shrimp, ghost shrimp and others. Arthropoda Crustacea Decapoda Dendrobranchiata – Prawns Caridea – Shrimp Astacidea – True lobsters and crayfish Thalassinidea - This group has recently -

Pinnixa Faba Class: Multicrustacea, Malacostraca, Eumalacostraca
Phylum: Arthropoda, Crustacea Pinnixa faba Class: Multicrustacea, Malacostraca, Eumalacostraca Order: Eucarida, Decapoda, Pleocyemata, Brachyura, The pea crab Eubrachyura, Thoracotremata Family: Pinnotheroidea, Pinnotheridae, Pinnothereliinae Description articulates at the inner proximal end of the Size: Female P. faba are much larger than merus. Exognath is with several joints and is males. Females are about 20 mm in width hidden (Rathbun 1918). while males are 10 mm wide (Fig. 1). Aver- Carapace: Carapace is smooth, round- age first true crab size is 1.54 mm (Pearce ed, swollen and oblong with no strong post- or 1966). anterolateral ridges. Carapace is 1.6–1.9 Color: Grayish tan with orange or rust col- times wider than long and sides are truncate, ored markings. Immature crabs are white, slope steeply and meet at an angle (Fig. 1) eggs orange and female cheliped tips are (Zmarzly 1992). Male carapaces sometimes white. Individuals are bright orange just have a vertical, compressed lobe at the anter- after molting (Pearce 1966). olateral angle (Fig. 4). General Morphology: The body of decapod Frontal Area: Narrow, slightly ad- crustaceans can be divided into the cepha- vanced in males and strong medial groove in lothorax (fused head and thorax) and abdo- females (Figs. 4, 1). men. They have a large plate-like carapace Teeth: No anterolateral teeth. dorsally, beneath which are five pairs of tho- Pereopods: The merus of males third racic appendages (see chelipeds and pere- walking leg is more than twice as long as wide opods) and three pairs of maxillipeds (see (Fig. 4). Dactyli of both sexes are short and mouthparts).