WO 2016/120843 Al 4 August 2016 (04.08.2016) P O P C T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rope Parasite” the Rope Parasite Parasites: Nearly Every Au�S�C Child I Ever Treated Proved to Carry a Significant Parasite Burden
Au#sm: 2015 Dietrich Klinghardt MD, PhD Infec4ons and Infestaons Chronic Infecons, Infesta#ons and ASD Infec4ons affect us in 3 ways: 1. Immune reac,on against the microbes or their metabolic products Treatment: low dose immunotherapy (LDI, LDA, EPD) 2. Effects of their secreted endo- and exotoxins and metabolic waste Treatment: colon hydrotherapy, sauna, intes4nal binders (Enterosgel, MicroSilica, chlorella, zeolite), detoxificaon with herbs and medical drugs, ac4vaon of detox pathways by solving underlying blocKages (methylaon, etc.) 3. Compe,,on for our micronutrients Treatment: decrease microbial load, consider vitamin/mineral protocol Lyme, Toxins and Epigene#cs • In 2000 I examined 10 au4s4c children with no Known history of Lyme disease (age 3-10), with the IgeneX Western Blot test – aer successful treatment. 5 children were IgM posi4ve, 3 children IgG, 2 children were negave. That is 80% of the children had clinical Lyme disease, none the history of a 4cK bite! • Why is it taking so long for au4sm-literate prac44oners to embrace the fact, that many au4s4c children have contracted Lyme or several co-infec4ons in the womb from an oVen asymptomac mother? Why not become Lyme literate also? • Infec4ons can be treated without the use of an4bio4cs, using liposomal ozonated essen4al oils, herbs, ozone, Rife devices, PEMF, colloidal silver, regular s.c injecons of artesunate, the Klinghardt co-infec4on cocKtail and more. • Symptomac infec4ons and infestaons are almost always the result of a high body burden of glyphosate, mercury and aluminum - against the bacKdrop of epigene4c injuries (epimutaons) suffered in the womb or from our ancestors( trauma, vaccine adjuvants, worK place related lead, aluminum, herbicides etc., electromagne4c radiaon exposures etc.) • Most symptoms are caused by a confused upregulated immune system (molecular mimicry) Toxins from a toxic environment enter our system through damaged boundaries and membranes (gut barrier, blood brain barrier, damaged endothelium, etc.). -

(12) Patent Application Publication (10) Pub. No.: US 2016/0220580 A1 Rubin Et Al
US 2016O220580A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0220580 A1 Rubin et al. (43) Pub. Date: Aug. 4, 2016 (54) SMALL MOLECULESCREENING FOR (60) Provisional application No. 61/497,708, filed on Jun. MOUSE SATELLITE CELL PROLIFERATION 16, 2011. (71) Applicant: PRESIDENT AND FELLOWS OF Publication Classification HARVARD COLLEGE, Cambridge, (51) Int. Cl. MA (US) A 6LX3/553 (2006.01) (72) Inventors: Lee L. Rubin, Wellesley, MA (US); A613 L/496 (2006.01) Amanda Gee, Alexandria, VA (US); A613 L/4439 (2006.01) Amy J. Wagers, Cambridge, MA (US) A613 L/404 (2006.01) (52) U.S. Cl. CPC ............. A6 IK3I/553 (2013.01); A61 K3I/404 (21) Appl. No.: 15/012,656 (2013.01); A61 K3I/496 (2013.01); A61 K 31/4439 (2013.01) (22) Filed: Feb. 1, 2016 (57) ABSTRACT The invention provides methods for inducing, enhancing or Related U.S. Application Data increasing satellite cell proliferation, and an assay for screen (63) Continuation-in-part of application No. 14/126,716, ing for a candidate compound for inducing, enhancing or filed on Jun. 13, 2014, now Pat. No. 9.248,185, filed as increasing satellite cell proliferation. Also provided are meth application No. PCT/US2012/042964 on Jun. 18, ods for repairing or regenerating a damaged muscle tissue of 2012. a Subject. Patent Application Publication Aug. 4, 2016 Sheet 1 of 44 US 2016/0220580 A1 FIG. A Patent Application Publication Aug. 4, 2016 Sheet 2 of 44 US 2016/0220580 A1 FIG. C. FIG. 2A Patent Application Publication Aug. -

Natural Modulators of Large-Conductance Calcium
Antonio Nardi1 Vincenzo Calderone2 Natural Modulators of Large-Conductance Silvio Chericoni3 Ivano Morelli3 Calcium-Activated Potassium Channels Review Abstract wards identifying new BK-modulating agents is proceeding with great impetus and is giving an ever-increasing number of Large-conductance calcium-activated potassium channels, also new molecules. Among these, also a handsome number of natur- known as BK or Maxi-K channels, occur in many types of cell, in- al BK-modulator compounds, belonging to different structural cluding neurons and myocytes, where they play an essential role classes, has appeared in the literature. The goal of this paper is in the regulation of cell excitability and function. These proper- to provide a possible simple classification of the broad structural ties open a possible role for BK-activators also called BK-open- heterogeneity of the natural BK-activating agents terpenes, phe- ers) and/or BK-blockers as effective therapeutic agents for differ- nols, flavonoids) and blockers alkaloids and peptides), and a ent neurological, urological, respiratory and cardiovascular dis- concise overview of their chemical and pharmacological proper- eases. The synthetic benzimidazolone derivatives NS004 and ties as well as potential therapeutic applications. NS1619 are the pioneer BK-activators and have represented the reference models which led to the design of several novel and Key words heterogeneous synthetic BK-openers, while very few synthetic Natural products ´ potassium channels ´ large-conductance cal- BK-blockers have been reported. Even today, the research to- cium-activated BK channels ´ BK-activators ´ BK-blockers 885 Introduction tracellular Ca2+ and membrane depolarisation, promoting a mas- sive outward flow of K+ ions and leading to a membrane hyper- Among the different factors exerting an influence on the activity polarisation, i.e., to a stabilisation of the cell [1]. -

(12) Patent Application Publication (10) Pub. No.: US 2010/0184685 A1 Zavala, JR
US 20100184685A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0184685 A1 Zavala, JR. et al. (43) Pub. Date: Jul. 22, 2010 (54) SYSTEMS AND METHODS FORTREATING Publication Classification POST OPERATIVE, ACUTE, AND CHRONIC (51) Int. Cl. PAIN USING AN INTRA-MUSCULAR A638/16 (2006.01) CATHETERADMINISTRATED A6IP 29/00 (2006.01) COMBINATION OF A LOCAL ANESTHETIC A6M 25/00 (2006.01) AND ANEUROTOXIN PROTEIN (52) U.S. Cl. ........................................... 514/12: 604/523 (57) ABSTRACT (76) Inventors: Gerardo Zavala, JR., San Antonio, TX (US); Gerardo Zavala, SR. Systems, and methods for the use of Such systems, are San Antonio, TX (US) described that allow for the administration of a combination of a Sustained release local anesthetic compound (such as bupivicaine) through a catheter based administration device Correspondence Address: and direct visualization or percutaneous injection of a neuro JACKSON WALKER LLP toxic protein compound (such as botulinum toxin) for post 901 MAIN STREET, SUITE 6000 operative and refractory treated muscle pain and discomfort DALLAS, TX 75202-3797 (US) in patients having undergone spinal Surgery and other muscle splitting or treatments aimed at improving muscle pain. The (21) Appl. No.: 12/689,381 systems utilize specific catheter-based administration proto cols and methods for placement of the catheter in association with muscles Surrounding the spine and other anatomical (22) Filed: Jan. 19, 2010 sites within the patient. The utilization of an initial bolus of a specific combination of medications (local anesthetic com Related U.S. Application Data pound and\or neurotoxic protein compound) followed by a dosage pump administration through the catheter is antici (60) Provisional application No. -

Venom‑Derived Peptide Modulators of Cation‑Selective Channels : Friend, Foe Or Frenemy
This document is downloaded from DR‑NTU (https://dr.ntu.edu.sg) Nanyang Technological University, Singapore. Venom‑derived peptide modulators of cation‑selective channels : friend, foe or frenemy Bajaj, Saumya; Han, Jingyao 2019 Bajaj, S., & Han, J. (2019). Venom‑Derived Peptide Modulators of Cation‑Selective Channels: Friend, Foe or Frenemy. Frontiers in Pharmacology, 10, 58‑. doi:10.3389/fphar.2019.00058 https://hdl.handle.net/10356/88522 https://doi.org/10.3389/fphar.2019.00058 © 2019 Bajaj and Han. This is an open‑access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms. Downloaded on 30 Sep 2021 04:20:36 SGT fphar-10-00058 February 23, 2019 Time: 18:29 # 1 MINI REVIEW published: 26 February 2019 doi: 10.3389/fphar.2019.00058 Venom-Derived Peptide Modulators of Cation-Selective Channels: Friend, Foe or Frenemy Saumya Bajaj*† and Jingyao Han† Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore Ion channels play a key role in our body to regulate homeostasis and conduct electrical signals. With the help of advances in structural biology, as well as the discovery of numerous channel modulators derived from animal toxins, we are moving toward a better understanding of the function and mode of action of ion channels. -

The Isolation, Structure, and Membrane Interactions Of
The isolation, structure, and membrane interactions of biologically active peptides A thesis submitted for the degree of doctor of philosophy By Patrick James Sherman B. Sc. (Hons.) from the Department of Chemistry The University of Adelaide June, 2012 Contents Acknowledgements viii Statement of originality x Abstract xi Abbreviations xiii Chapter 1 Biologically active peptides 1 1.1 Synopsis 1 1.2 Peptide Biosynthesis 2 1.3 Anuran secretions 4 1.3.1 Collection of anuran secretion 5 1.3.2 Australian anuran peptides 7 1.4 Scorpion venoms 13 1.4.1 Collection of scorpion venom 14 1.4.2 Scorpion peptides 15 Chapter 2 Methodology I – Mass Spectrometry 20 2.1 Mass Spectrometry 20 2.2 The Q-TOF2 Mass Spectrometer 21 2.2.1 The Quadrupole analyser 22 2.2.2 The Hexapole Collision Cell 23 2.2.3 The Time of Flight Sector 24 2.3 Electrospray ionisation 25 2.4 Peptide sequence determination 26 2.4.1 High Performance Liquid Chromatography 27 2.4.2 Positive ion fragmentation 27 2.4.3 Negative ion fragmentation 28 2.4.4 Edman Sequencing 31 - ii - Chapter 3 Methodology II – Nuclear Magnetic Resonance Spectroscopy 33 3.1 Nuclear magnetic resonance spectroscopy of peptides in solution 33 3.1.1 Principles of nuclear magnetic resonance spectroscopy 34 3.1.2 One-dimensional NMR spectroscopy 36 3.1.3 Two-dimensional NMR spectroscopy 40 3.1.3.1 Correlation NMR spectroscopy 41 3.1.3.2 Total correlation NMR spectroscopy 44 3.1.3.3 Nuclear Overhauser effect NMR spectroscopy 45 3.1.4 Chemical shift Assignment 46 3.1.5 NOE Connectivities 48 3.1.6 Secondary shifts -

(12) Patent Application Publication (10) Pub. No.: US 2014/0088056A1 Ye Et Al
US 20140O88056A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0088056A1 Ye et al. (43) Pub. Date: Mar. 27, 2014 (54) CARDIAC GLYCOSIDES ARE POTENT Publication Classification INHIBITORS OF INTERFERON-BETA GENE EXPRESSION (51) Int. Cl. A613 L/585 (2006.01) A 6LX3/59 (2006.01) (75) Inventors: Junqiang Ye, Fort Lee, NJ (US); A613 L/7 (2006.01) Shuibing Chen, Pelham, NY (US); Tom A 6LX3 L/505 (2006.01) Maniatis, New York, NY (US) A613 L/407 (2006.01) A63L/36 (2006.01) (52) U.S. Cl. (73) Assignee: PRESIDENT AND FELLOWS OF CPC ............. A6 IK3I/585 (2013.01); A61 K3I/407 HARVARD COLLEGE, Cambridge, (2013.01); A61 K3I/136 (2013.01); A61 K MA (US) 3 1/17 (2013.01); A61K3I/505 (2013.01); A6 IK3I/519 (2013.01) (21) Appl. No.: 13/876,795 USPC ........... 514/175: 514/410; 514/656; 514/597; (22) PCT Filed: Sep. 28, 2011 514/256; 514/264.11: 435/375; 435/184 (57) ABSTRACT (86). PCT No.: The invention provides for a method of inhibiting interferon S371 (c)(1), beta gene expression and/or reducing the level of interferon (2), (4) Date: Nov. 25, 2013 beta in a cell by contacting the cell with a Na", Ca", or K" ion-channel modulator. The invention also provides for a method of treating a disease or disorder characterized by Related U.S. Application Data elevated interferon beta levels or elevated levels of interferon (60) Provisional application No. 61/387,407, filed on Sep. beta gene expression. Additionally, the invention provides a 28, 2010. -

Ion Channel Pharmacology
Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics Ion Channel Pharmacology Diana Conte Camerino, Domenico Tricarico, and Jean-François Desaphy Pharmacology Division, Department of Pharmacobiology, School of Pharmacy, University of Bari, Bari, Italy Summary: Because ion channels are involved in many cellular tions have demonstrated that channel mutations can either in- processes, drugs acting on ion channels have long been used for crease or decrease affinity for the drug, modifying its potential the treatment of many diseases, especially those affecting elec- therapeutic effect. Together with the discovery of channel gene trically excitable tissues. The present review discusses the phar- polymorphisms that may affect drug pharmacodynamics, these macology of voltage-gated and neurotransmitter-gated ion findings highlight the need for pharmacogenetic research to channels involved in neurologic diseases, with emphasis on allow identification of drugs with more specific effects on neurologic channelopathies. With the discovery of ion chan- channel isoforms or mutants, to increase efficacy and reduce nelopathies, the therapeutic value of many basic drugs targeting side effects. With a greater understanding of channel genetics, ion channels has been confirmed. The understanding of the structure, and function, together with the identification of novel genotype–phenotype relationship has highlighted possible ac- primary and secondary channelopathies, the number of ion tion mechanisms of other empirically used drugs. Moreover, channel drugs for neurologic channelopathies will increase sub- other ion channels have been pinpointed as potential new Key Words: drug targets. With regards to therapy of channelopathies, ex- stantially. Voltage-gated, neurotransmitter-gated, perimental investigations of the intimate drug–channel interac- ion channel, drug therapy, channelopathy, pharmacogenetics. -

(12) United States Patent (10) Patent No.: US 9.248,185 B2 Rubin Et Al
USOO9248185B2 (12) United States Patent (10) Patent No.: US 9.248,185 B2 Rubin et al. (45) Date of Patent: Feb. 2, 2016 (54) METHODS OF INCREASING SATELLITE (2013.01); A61 K3I/485 (2013.01); A61 K CELL PROLIFERATION 3 I/553 (2013.01); A61 K3I/58 (2013.01); A61K3I/7076 (2013.01); G0IN33/5044 (75) Inventors: Lee L. Rubin, Wellesley, MA (US); (2013.01); C07D498/22 (2013.01) Amanda Gee, Alexandria, VA (US); (58) Field of Classification Search Amy J. Wagers, Cambridge, MA (US) None (73) Assignee: President and Fellows of Harvard See application file for complete search history. College, Cambridge, MA (US) (56) References Cited (*) Notice: Subject to any disclaimer, the term of this U.S. PATENT DOCUMENTS patent is extended or adjusted under 35 U.S.C. 154(b) by 0 days. 2003/018151.0 A1* 9, 2003 Baker et al. ................... 514,432 2005/0281788 A1 12/2005 de Bari et al. (21) Appl. No.: 14/126,716 2010.0048534 A1 2/2010 Dziki et al. .............. 514, 21108 (22) PCT Filed: Jun. 18, 2012 FOREIGN PATENT DOCUMENTS (86). PCT No.: PCT/US2O12/042964 RU 2368398 9, 2009 S371 (c)(1), OTHER PUBLICATIONS (2), (4) Date: Jun. 13, 2014 Shea et al (2010. Cell Stem Cell. 6: 117-129).* Strocket al., 2003. Cancer Research. 63:5559-5563.* (87) PCT Pub. No.: WO2012/174537 Mulligan et al., 2004. Nature Reviews: Cancer, 14: 173-186.* PCT Pub. Date: Dec. 20, 2012 Carlson, et al. “Relative roles of TGF-B1 and Wnt in the systemic regulation and aging of Satellite cell responses'. -
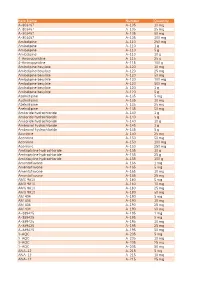
Item Name Catalog Number Quantity A-803467 A-105 10 Mg A
Catalog Item Name Number Quantity A-803467 A-105 10 mg A-803467 A-105 25 mg A-803467 A-105 50 mg A-803467 A-105 100 mg Amlodipine A-110 250 mg Amlodipine A-110 1 g Amlodipine A-110 5 g Amlodipine A-110 10 g 4-Aminopyridine A-115 25 g 4-Aminopyridine A-115 100 g Amlodipine besylate A-120 10 mg Amlodipine besylate A-120 25 mg Amlodipine besylate A-120 50 mg Amlodipine besylate A-120 100 mg Amlodipine besylate A-120 500 mg Amlodipine besylate A-120 1 g Amlodipine besylate A-120 5 g Azelnidipine A-135 5 mg Azelnidipine A-135 10 mg Azelnidipine A-135 25 mg Azelnidipine A-135 50 mg Amiloride hydrochloride A-140 1 g Amiloride hydrochloride A-140 5 g Amiloride hydrochloride A-140 10 g Ambroxol hydrochloride A-145 1 g Ambroxol hydrochloride A-145 5 g Aconitine A-150 25 mg Aconitine A-150 50 mg Aconitine A-150 100 mg Aconitine A-150 250 mg Amitriptyline hydrochloride A-155 10 g Amitriptyline hydrochloride A-155 25 g Amitriptyline hydrochloride A-155 100 g Amentoflavone A-165 1 mg Amentoflavone A-165 5 mg Amentoflavone A-165 10 mg Amentoflavone A-165 25 mg AMG 9810 A-180 5 mg AMG 9810 A-180 10 mg AMG 9810 A-180 25 mg AMG 9810 A-180 50 mg AM 404 A-190 5 mg AM 404 A-190 10 mg AM 404 A-190 25 mg AM 404 A-190 50 mg A-889425 A-195 1 mg A-889425 A-195 5 mg A-889425 A-195 10 mg A-889425 A-195 25 mg A-889425 A-195 50 mg 3-AQC A-205 5 mg 3-AQC A-205 10 mg 3-AQC A-205 25 mg 3-AQC A-205 50 mg ANA-12 A-215 5 mg ANA-12 A-215 10 mg ANA-12 A-215 25 mg ANA-12 A-215 50 mg ANA-12 A-215 100 mg A 967079 A-225 5 mg A 967079 A-225 10 mg A 967079 A-225 25 mg A 967079 -

The Slo(W) Path to Identifying the Mitochondrial Channels Responsible for Ischemic Protection
Biochemical Journal (2017) 474 2067–2094 DOI: 10.1042/BCJ20160623 Review Article The Slo(w) path to identifying the mitochondrial channels responsible for ischemic protection Charles Owen Smith1, Keith Nehrke2 and Paul S. Brookes3 1Department of Biochemistry, University of Rochester Medical Center, Rochester, NY, U.S.A.; 2Department of Medicine, University of Rochester Medical Center, Rochester, NY, U.S.A.; and 3Department of Anesthesiology, University of Rochester Medical Center, 601 Elmwood Avenue, Rochester, NY 14642, U.S.A. Correspondence: Paul S. Brookes ([email protected]) Mitochondria play an important role in tissue ischemia and reperfusion (IR) injury, with energetic failure and the opening of the mitochondrial permeability transition pore being the major causes of IR-induced cell death. Thus, mitochondria are an appropriate focus for strategies to protect against IR injury. Two widely studied paradigms of IR protection, particularly in the field of cardiac IR, are ischemic preconditioning (IPC) and volatile anes- thetic preconditioning (APC). While the molecular mechanisms recruited by these protect- ive paradigms are not fully elucidated, a commonality is the involvement of mitochondrial K+ channel opening. In the case of IPC, research has focused on a mitochondrial + ATP-sensitive K channel (mitoKATP), but, despite recent progress, the molecular identity of this channel remains a subject of contention. In the case of APC, early research sug- gested the existence of a mitochondrial large-conductance K+ (BK, big conductance of potassium) channel encoded by the Kcnma1 gene, although more recent work has shown that the channel that underlies APC is in fact encoded by Kcnt2. In this review, we discuss both the pharmacologic and genetic evidence for the existence and identity of mitochondrial K+ channels, and the role of these channels both in IR protection and in regulating normal mitochondrial function. -

Venom Peptides and Their Mimetics As Potential Drugs
Venom Peptides and their Mimetics as Potential Drugs Oren Bogin, Ph.D. Venomous creatures have a sophisticated mechanism for prey capture which includes a vast array of biologically-active compounds, such as enzymes, proteins, peptides and small molecular weight compounds. These substances target an immense number of receptors and membrane proteins with high affinity, selectivity and potency, and can serve as potential drugs or scaffolds for drug design. Introduction Pain channels has been established13 (please refer to the article “Contribution of Ion Channels in Pain Sensation” in this Modulator issue). Pain A large number of organisms produce and secrete Recurrent pain was recently termed the “silent signals can be blocked at a number of sites along venoms to defend themselves and to capture epidemic”, since one out of six people in the the pain pathway (See Figure). The large list of prey. Venom is a rich source of biochemically western world suffers from pain, costing the neurotransmitters and receptors identified along active enzymes, proteins, peptides and low American public alone approximately $100 the pain pathway indicate that there may be many molecular weight substances. Toxins isolated billion each year in health care, compensation, therapeutic possibilities for the pharmacological from the venom either inhibit or activate a and litigation.11,12 Chronic pain is associated control of the transmission of nociceptive vast number of targets such as ion channels, with conditions such as back injury, migraine information to the brain. acetylcholine receptors, acetylcholinesterase, headaches, arthritis, herpes zoster, diabetic membranes, coagulant/anticoagulant pathways, neuropathy, temporomandibular joint syndrome, Voltage-Gated Ca2+ Channels (VGCC) and metalloproteases, with high selectivity and cancer.