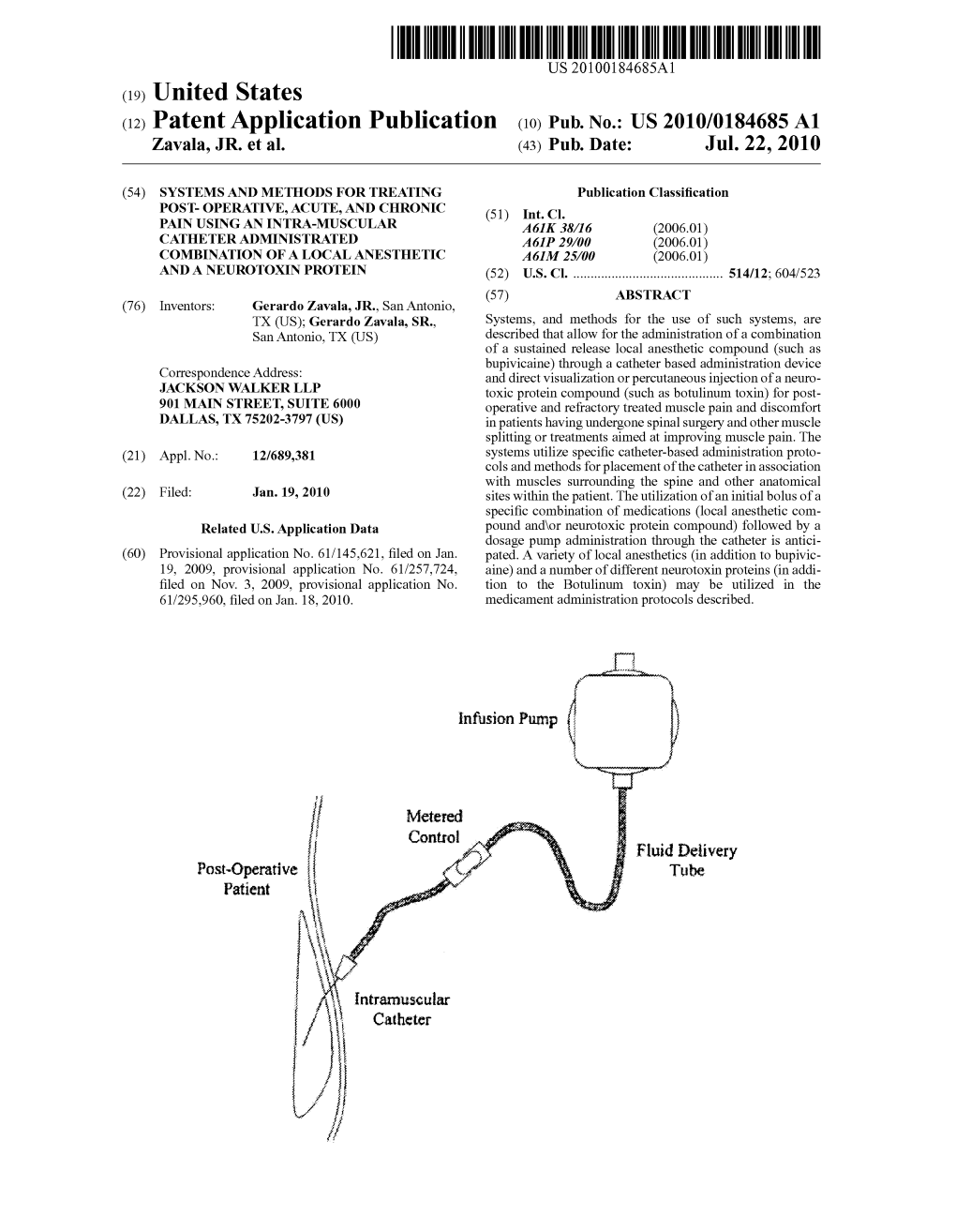
(12) Patent Application Publication (10) Pub. No.: US 2010/0184685 A1 Zavala, JR
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

KEGG Orthology-Based Annotation of the Predicted
Dunlap et al. BMC Genomics 2013, 14:509 http://www.biomedcentral.com/1471-2164/14/509 DATABASE Open Access KEGG orthology-based annotation of the predicted proteome of Acropora digitifera: ZoophyteBase - an open access and searchable database of a coral genome Walter C Dunlap1,2, Antonio Starcevic4, Damir Baranasic4, Janko Diminic4, Jurica Zucko4, Ranko Gacesa4, Madeleine JH van Oppen1, Daslav Hranueli4, John Cullum5 and Paul F Long2,3* Abstract Background: Contemporary coral reef research has firmly established that a genomic approach is urgently needed to better understand the effects of anthropogenic environmental stress and global climate change on coral holobiont interactions. Here we present KEGG orthology-based annotation of the complete genome sequence of the scleractinian coral Acropora digitifera and provide the first comprehensive view of the genome of a reef-building coral by applying advanced bioinformatics. Description: Sequences from the KEGG database of protein function were used to construct hidden Markov models. These models were used to search the predicted proteome of A. digitifera to establish complete genomic annotation. The annotated dataset is published in ZoophyteBase, an open access format with different options for searching the data. A particularly useful feature is the ability to use a Google-like search engine that links query words to protein attributes. We present features of the annotation that underpin the molecular structure of key processes of coral physiology that include (1) regulatory proteins of -

An in Vivo Examination of the Differences Between Rapid
www.nature.com/scientificreports OPEN An in vivo examination of the diferences between rapid cardiovascular collapse and prolonged hypotension induced by snake venom Rahini Kakumanu1, Barbara K. Kemp-Harper1, Anjana Silva 1,2, Sanjaya Kuruppu3, Geofrey K. Isbister 1,4 & Wayne C. Hodgson1* We investigated the cardiovascular efects of venoms from seven medically important species of snakes: Australian Eastern Brown snake (Pseudonaja textilis), Sri Lankan Russell’s viper (Daboia russelii), Javanese Russell’s viper (D. siamensis), Gaboon viper (Bitis gabonica), Uracoan rattlesnake (Crotalus vegrandis), Carpet viper (Echis ocellatus) and Puf adder (Bitis arietans), and identifed two distinct patterns of efects: i.e. rapid cardiovascular collapse and prolonged hypotension. P. textilis (5 µg/kg, i.v.) and E. ocellatus (50 µg/kg, i.v.) venoms induced rapid (i.e. within 2 min) cardiovascular collapse in anaesthetised rats. P. textilis (20 mg/kg, i.m.) caused collapse within 10 min. D. russelii (100 µg/kg, i.v.) and D. siamensis (100 µg/kg, i.v.) venoms caused ‘prolonged hypotension’, characterised by a persistent decrease in blood pressure with recovery. D. russelii venom (50 mg/kg and 100 mg/kg, i.m.) also caused prolonged hypotension. A priming dose of P. textilis venom (2 µg/kg, i.v.) prevented collapse by E. ocellatus venom (50 µg/kg, i.v.), but had no signifcant efect on subsequent addition of D. russelii venom (1 mg/kg, i.v). Two priming doses (1 µg/kg, i.v.) of E. ocellatus venom prevented collapse by E. ocellatus venom (50 µg/kg, i.v.). B. gabonica, C. vegrandis and B. -

Toxicology in Antiquity
TOXICOLOGY IN ANTIQUITY Other published books in the History of Toxicology and Environmental Health series Wexler, History of Toxicology and Environmental Health: Toxicology in Antiquity, Volume I, May 2014, 978-0-12-800045-8 Wexler, History of Toxicology and Environmental Health: Toxicology in Antiquity, Volume II, September 2014, 978-0-12-801506-3 Wexler, Toxicology in the Middle Ages and Renaissance, March 2017, 978-0-12-809554-6 Bobst, History of Risk Assessment in Toxicology, October 2017, 978-0-12-809532-4 Balls, et al., The History of Alternative Test Methods in Toxicology, October 2018, 978-0-12-813697-3 TOXICOLOGY IN ANTIQUITY SECOND EDITION Edited by PHILIP WEXLER Retired, National Library of Medicine’s (NLM) Toxicology and Environmental Health Information Program, Bethesda, MD, USA Academic Press is an imprint of Elsevier 125 London Wall, London EC2Y 5AS, United Kingdom 525 B Street, Suite 1650, San Diego, CA 92101, United States 50 Hampshire Street, 5th Floor, Cambridge, MA 02139, United States The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, United Kingdom Copyright r 2019 Elsevier Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangements with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions. This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). -

Ion Channels 3 1
r r r Cell Signalling Biology Michael J. Berridge Module 3 Ion Channels 3 1 Module 3 Ion Channels Synopsis Ion channels have two main signalling functions: either they can generate second messengers or they can function as effectors by responding to such messengers. Their role in signal generation is mainly centred on the Ca2 + signalling pathway, which has a large number of Ca2+ entry channels and internal Ca2+ release channels, both of which contribute to the generation of Ca2 + signals. Ion channels are also important effectors in that they mediate the action of different intracellular signalling pathways. There are a large number of K+ channels and many of these function in different + aspects of cell signalling. The voltage-dependent K (KV) channels regulate membrane potential and + excitability. The inward rectifier K (Kir) channel family has a number of important groups of channels + + such as the G protein-gated inward rectifier K (GIRK) channels and the ATP-sensitive K (KATP) + + channels. The two-pore domain K (K2P) channels are responsible for the large background K current. Some of the actions of Ca2 + are carried out by Ca2+-sensitive K+ channels and Ca2+-sensitive Cl − channels. The latter are members of a large group of chloride channels and transporters with multiple functions. There is a large family of ATP-binding cassette (ABC) transporters some of which have a signalling role in that they extrude signalling components from the cell. One of the ABC transporters is the cystic − − fibrosis transmembrane conductance regulator (CFTR) that conducts anions (Cl and HCO3 )and contributes to the osmotic gradient for the parallel flow of water in various transporting epithelia. -

Rope Parasite” the Rope Parasite Parasites: Nearly Every Au�S�C Child I Ever Treated Proved to Carry a Significant Parasite Burden
Au#sm: 2015 Dietrich Klinghardt MD, PhD Infec4ons and Infestaons Chronic Infecons, Infesta#ons and ASD Infec4ons affect us in 3 ways: 1. Immune reac,on against the microbes or their metabolic products Treatment: low dose immunotherapy (LDI, LDA, EPD) 2. Effects of their secreted endo- and exotoxins and metabolic waste Treatment: colon hydrotherapy, sauna, intes4nal binders (Enterosgel, MicroSilica, chlorella, zeolite), detoxificaon with herbs and medical drugs, ac4vaon of detox pathways by solving underlying blocKages (methylaon, etc.) 3. Compe,,on for our micronutrients Treatment: decrease microbial load, consider vitamin/mineral protocol Lyme, Toxins and Epigene#cs • In 2000 I examined 10 au4s4c children with no Known history of Lyme disease (age 3-10), with the IgeneX Western Blot test – aer successful treatment. 5 children were IgM posi4ve, 3 children IgG, 2 children were negave. That is 80% of the children had clinical Lyme disease, none the history of a 4cK bite! • Why is it taking so long for au4sm-literate prac44oners to embrace the fact, that many au4s4c children have contracted Lyme or several co-infec4ons in the womb from an oVen asymptomac mother? Why not become Lyme literate also? • Infec4ons can be treated without the use of an4bio4cs, using liposomal ozonated essen4al oils, herbs, ozone, Rife devices, PEMF, colloidal silver, regular s.c injecons of artesunate, the Klinghardt co-infec4on cocKtail and more. • Symptomac infec4ons and infestaons are almost always the result of a high body burden of glyphosate, mercury and aluminum - against the bacKdrop of epigene4c injuries (epimutaons) suffered in the womb or from our ancestors( trauma, vaccine adjuvants, worK place related lead, aluminum, herbicides etc., electromagne4c radiaon exposures etc.) • Most symptoms are caused by a confused upregulated immune system (molecular mimicry) Toxins from a toxic environment enter our system through damaged boundaries and membranes (gut barrier, blood brain barrier, damaged endothelium, etc.). -

Comparative Studies of the Venom of a New Taipan Species, Oxyuranus Temporalis, with Other Members of Its Genus
Toxins 2014, 6, 1979-1995; doi:10.3390/toxins6071979 OPEN ACCESS toxins ISSN 2072-6651 www.mdpi.com/journal/toxins Article Comparative Studies of the Venom of a New Taipan Species, Oxyuranus temporalis, with Other Members of Its Genus Carmel M. Barber 1, Frank Madaras 2, Richard K. Turnbull 3, Terry Morley 4, Nathan Dunstan 5, Luke Allen 5, Tim Kuchel 3, Peter Mirtschin 2 and Wayne C. Hodgson 1,* 1 Monash Venom Group, Department of Pharmacology, Faculty of Medicine, Nursing and Health Sciences, Monash University, Clayton, Victoria 3168, Australia; E-Mail: [email protected] 2 Venom Science Pty Ltd, Tanunda, South Australia 5352, Australia; E-Mails: [email protected] (F.M.); [email protected] (P.M.) 3 SA Pathology, IMVS Veterinary Services, Gilles Plains, South Australia 5086, Australia; E-Mails: [email protected] (R.K.T.); [email protected] (T.K.) 4 Adelaide Zoo, Adelaide, South Australia 5000, Australia; E-Mail: [email protected] 5 Venom Supplies, Tanunda, South Australia, South Australia 5352, Australia; E-Mails: [email protected] (N.D.); [email protected] (L.A.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +61-3-9905-4861; Fax: +61-3-9905-2547. Received: 5 May 2014; in revised form: 11 June 2014 / Accepted: 16 June 2014 / Published: 2 July 2014 Abstract: Taipans are highly venomous Australo-Papuan elapids. A new species of taipan, the Western Desert Taipan (Oxyuranus temporalis), has been discovered with two specimens housed in captivity at the Adelaide Zoo. This study is the first investigation of O. -

Androctonus Mauretanicus Mauretanicus
Hindawi Publishing Corporation Journal of Toxicology Volume 2012, Article ID 103608, 9 pages doi:10.1155/2012/103608 Review Article Potassium Channels Blockers from the Venom of Androctonus mauretanicus mauretanicus Marie-France Martin-Eauclaire and Pierre E. Bougis Aix-Marseille University, CNRS, UMR 7286, CRN2M, Facult´edeM´edecine secteur Nord, CS80011, Boulevard Pierre Dramard, 13344 Marseille Cedex 15, France Correspondence should be addressed to Marie-France Martin-Eauclaire, [email protected] and Pierre E. Bougis, [email protected] Received 2 February 2012; Accepted 16 March 2012 Academic Editor: Maria Elena de Lima Copyright © 2012 M.-F. Martin-Eauclaire and P. E. Bougis. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. K+ channels selectively transport K+ ions across cell membranes and play a key role in regulating the physiology of excitable and nonexcitable cells. Their activation allows the cell to repolarize after action potential firing and reduces excitability, whereas channel inhibition increases excitability. In eukaryotes, the pharmacology and pore topology of several structural classes of K+ channels have been well characterized in the past two decades. This information has come about through the extensive use of scorpion toxins. We have participated in the isolation and in the characterization of several structurally distinct families of scorpion toxin peptides exhibiting different K+ channel blocking functions. In particular, the venom from the Moroccan scorpion Androctonus ffi + + mauretanicus mauretanicus provided several high-a nity blockers selective for diverse K channels (SKCa,Kv4.x, and Kv1.x K channel families). -

Biochemical Characterization of Phospholipase A2 (Trimorphin) from the Venom of the Sonoran Lyre Snake Trimorphodon Biscutatus Lambda (Family Colubridae)
Toxicon 44 (2004) 27–36 www.elsevier.com/locate/toxicon Biochemical characterization of phospholipase A2 (trimorphin) from the venom of the Sonoran Lyre Snake Trimorphodon biscutatus lambda (family Colubridae) Ping Huang, Stephen P. Mackessy* Department of Biological Sciences, University of Northern Colorado, 501 20th St., CB 92, Greeley, CO 80639-0017, USA Received 3 December 2003; accepted 23 March 2004 Available online 18 May 2004 Abstract Phospholipases A2 (PLA2), common venom components and bioregulatory enzymes, have been isolated and sequenced from many snake venoms, but never from the venom (Duvernoy’s gland secretion) of colubrid snakes. We report for the first time the purification, biochemical characterization and partial sequence of a PLA2 (trimorphin) from the venom of a colubrid snake, Trimorphodon biscutatus lambda (Sonoran Lyre Snake). Specific phospholipase activity of the purified PLA2 was confirmed by enzyme assays. The molecular weight of the enzyme has been determined by SDS-PAGE and mass spectrometry to be 13,996 kDa. The sequence of 50 amino acid residues from the N-terminal has been identified and shows a high degree of sequence homology to the type IA PLA2s, especially the Asp-49 enzymes. The Cys-11 residue, characteristic of the group IA 2þ PLA2s, and the Ca binding loop residues (Tyr-28, Gly-30, Gly-32, and Asp-49) are conserved. In addition, the His-48 residue, a key component of the active site, is also conserved in trimorphin. The results of phylogenetic analysis on the basis of amino acid sequence homology demonstrate that trimorphin belongs to the type IA family, and it appears to share a close evolutionary relationship with the PLA2s from hydrophiine elapid snakes (sea snakes and Australian venomous snakes). -

Neurotoxicity in Snakebite—The Limits of Our Knowledge
Review Neurotoxicity in Snakebite—The Limits of Our Knowledge Udaya K. Ranawaka1*, David G. Lalloo2, H. Janaka de Silva1 1 Faculty of Medicine, University of Kelaniya, Ragama, Sri Lanka, 2 Liverpool School of Tropical Medicine, Liverpool, United Kingdom been well described with pit vipers (family Viperidae, subfamily Abstract: Snakebite is classified by the WHO as a Crotalinae) such as rattlesnakes (Crotalus spp.) [58–67]. Although neglected tropical disease. Envenoming is a significant considered relatively less common with true vipers (family public health problem in tropical and subtropical regions. Viperidae, subfamily Viperinae), neurotoxicity is well recognized Neurotoxicity is a key feature of some envenomings, and in envenoming with Russell’s viper (Daboia russelii) in Sri Lanka and there are many unanswered questions regarding this South India [9,68–75], the asp viper (Vipera aspis) [76–82], the manifestation. Acute neuromuscular weakness with respi- adder (Vipera berus) [83–85], and the nose-horned viper (Vipera ratory involvement is the most clinically important ammodytes) [86,87]. neurotoxic effect. Data is limited on the many other acute neurotoxic manifestations, and especially delayed Acute neuromuscular paralysis is the main type of neurotoxicity neurotoxicity. Symptom evolution and recovery, patterns and is an important cause of morbidity and mortality related to of weakness, respiratory involvement, and response to snakebite. Mechanical ventilation, intensive care, antivenom antivenom and acetyl cholinesterase inhibitors are vari- treatment, other ancillary care, and prolonged hospital stays all able, and seem to depend on the snake species, type of contribute to a significant cost of provision of care. And ironically, neurotoxicity, and geographical variations. Recent data snakebite is common in resource-poor countries that can ill afford have challenged the traditional concepts of neurotoxicity such treatment costs. -

(12) Patent Application Publication (10) Pub. No.: US 2016/0220580 A1 Rubin Et Al
US 2016O220580A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0220580 A1 Rubin et al. (43) Pub. Date: Aug. 4, 2016 (54) SMALL MOLECULESCREENING FOR (60) Provisional application No. 61/497,708, filed on Jun. MOUSE SATELLITE CELL PROLIFERATION 16, 2011. (71) Applicant: PRESIDENT AND FELLOWS OF Publication Classification HARVARD COLLEGE, Cambridge, (51) Int. Cl. MA (US) A 6LX3/553 (2006.01) (72) Inventors: Lee L. Rubin, Wellesley, MA (US); A613 L/496 (2006.01) Amanda Gee, Alexandria, VA (US); A613 L/4439 (2006.01) Amy J. Wagers, Cambridge, MA (US) A613 L/404 (2006.01) (52) U.S. Cl. CPC ............. A6 IK3I/553 (2013.01); A61 K3I/404 (21) Appl. No.: 15/012,656 (2013.01); A61 K3I/496 (2013.01); A61 K 31/4439 (2013.01) (22) Filed: Feb. 1, 2016 (57) ABSTRACT The invention provides methods for inducing, enhancing or Related U.S. Application Data increasing satellite cell proliferation, and an assay for screen (63) Continuation-in-part of application No. 14/126,716, ing for a candidate compound for inducing, enhancing or filed on Jun. 13, 2014, now Pat. No. 9.248,185, filed as increasing satellite cell proliferation. Also provided are meth application No. PCT/US2012/042964 on Jun. 18, ods for repairing or regenerating a damaged muscle tissue of 2012. a Subject. Patent Application Publication Aug. 4, 2016 Sheet 1 of 44 US 2016/0220580 A1 FIG. A Patent Application Publication Aug. 4, 2016 Sheet 2 of 44 US 2016/0220580 A1 FIG. C. FIG. 2A Patent Application Publication Aug. -

Structure±Function Properties of Venom Components from Australian Elapids
PERGAMON Toxicon 37 (1999) 11±32 Review Structure±function properties of venom components from Australian elapids Bryan Grieg Fry * Peptide Laboratory, Centre for Drug Design and Development, University of Queensland, St. Lucia, Qld, 4072, Australia Received 9 December 1997; accepted 4 March 1998 Abstract A comprehensive review of venom components isolated thus far from Australian elapids. Illustrated is that a tremendous structural homology exists among the components but this homology is not representative of the functional diversity. Further, the review illuminates the overlooked species and areas of research. # 1998 Elsevier Science Ltd. All rights reserved. 1. Introduction Australian elapids are well known to be the most toxic in the world, with all of the top ten and nineteen of the top 25 elapids with known LD50s residing exclusively on this continent (Broad et al., 1979). Thus far, three main types of venom components have been characterised from Australian elapids: prothrombin activating enzymes; lipases with a myriad of potent activities; and powerful peptidic neurotoxins. Many species have the prothrombin activating enzymes in their venoms, the vast majority contain phospholipase A2s and all Australian elapid venoms are suspected to contain peptidic neurotoxins. In addition to the profound neurological eects such as disorientation, ¯accid paralysis and respiratory failure, characteristic of bites by many species of Australian elapids is hemorrhaging and incoagulable blood. As a result, these elapids can be divided into two main classes: species with procoagulant venom (Table 1) and species with non-procoagulant venoms (Table 2) (Tan and * Author to whom correspondence should be addressed. 0041-0101/98/$ - see front matter # 1998 Elsevier Science Ltd. -

In the Molecular Evolution of Snake Venom Proteins Robin Doley National University of Singapore
University of Northern Colorado Scholarship & Creative Works @ Digital UNC School of Biological Sciences Faculty Publications School of Biological Sciences 2009 Role of Accelerated Segment Switch in Exons to Alter Targeting (Asset) in the Molecular Evolution of Snake Venom Proteins Robin Doley National University of Singapore Stephen P. Mackessy University of Northern Colorado R. Manjunatha Kini National University of Singapore Follow this and additional works at: http://digscholarship.unco.edu/biofacpub Part of the Biology Commons Recommended Citation Doley, Robin; Mackessy, Stephen P.; and Kini, R. Manjunatha, "Role of Accelerated Segment Switch in Exons to Alter Targeting (Asset) in the Molecular Evolution of Snake Venom Proteins" (2009). School of Biological Sciences Faculty Publications. 7. http://digscholarship.unco.edu/biofacpub/7 This Article is brought to you for free and open access by the School of Biological Sciences at Scholarship & Creative Works @ Digital UNC. It has been accepted for inclusion in School of Biological Sciences Faculty Publications by an authorized administrator of Scholarship & Creative Works @ Digital UNC. For more information, please contact [email protected]. BMC Evolutionary Biology BioMed Central Research article Open Access Role of accelerated segment switch in exons to alter targeting (ASSET) in the molecular evolution of snake venom proteins Robin Doley1, Stephen P Mackessy2 and R Manjunatha Kini*1 Address: 1Protein Science Laboratory, Department of Biological Sciences, National University of