Supplementary Information Doi: 10.1038/Nclimate1663
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -
The Butterflies of Mississippi Supplement No
Journal of the Lepidopterists' Society 39(2), 1985, 134-138 THE BUTTERFLIES OF MISSISSIPPI SUPPLEMENT NO. 31 BRYANT MATHER2 AND KATHARINE MATHER 213 Mt. Salus Road, Clinton, Mississippi 39056 ABSTRACT. An annotated list of Mississippi butterflies is presented. This updated version is the fifth such list published. Six names additional to the previous lists have been included. Of the five published lists of Mississippi butterflies, this is the first to use the names and arrangement of Miller and Brown (1981) as amend ed by them in Hodges (Editor) (1983). It includes six names not in cluded in the fourth list (Mather & Mather, 1976). The growth rate has dropped to fewer than one per year as indicated in Table 1 below: TABLE 1. Published lists of Mississippi butterflies, showing rate of increase in the addition of names previously unrecorded from the state. Time Names added List Reference Names interval Names added per year 1 Weed (1894) 53 2 Hutchins (1933) 73 39 20 0.5 3 M. & M. (1958) 122 25 49 1.9 4 M. & M. (1976) 144 18 22 1.2 5 M. & M. (1984) 150 8 6 0.75 In 1958 we expressed the opinion that the list would grow to include about 160 names. We also said, "there may be cases in which the Mississippi representatives of a given species represent more than one population; if so, we do not believe that we as yet have adequate data to support such a conclusion." Now we do. Our reasons for adding the six names are summarized below. 1. -
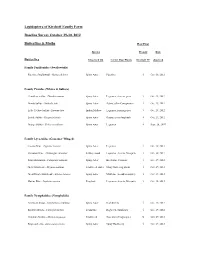
Butterflies and Moths List
Lepidoptera of Kirchoff Family Farm Baseline Survey October 29-30, 2012 Butterflies & Moths Host Plant Species Present Date Butterflies Observed On Larval Host Plants Kirchoff FF observed Family Papilionidae (Swallowtails) Pipevine Swallowtail - Battus philenor Spiny Aster Pipevine √ Oct. 30, 2012 Family Pieridae (Whites & Sulfurs) Cloudless Sulfur - Phoebis sennae Spiny Aster Legumes, clovers, peas √ Oct. 29, 2012 Dainty Sulfur - Nathalis iole Spiny Aster Asters, other Com;posites √ Oct. 29, 2012 Little Yellow Sulfur - Eurema lisa Indian Mallow Legumes, partridge pea √ Oct. 29, 2012 Lyside Sulfur - Kirgonia lyside Spiny Aster Guayacan or Soapbush √ Oct. 29, 2012 Orange Sulfur - Colias eurytheme Spiny Aster Legumes √ Sept. 26, 2007 Family Lycaenidae (Gossamer Winged) Cassius Blue - Leptotes cassius Spiny Aster Legumes √ Oct. 30, 2012 Ceraunus Blue - Hemiargus ceraunus Kidney wood Legumes- Acacia, Mesquite √ Oct. 30, 2012 Fatal Metalmark - Calephelis nemesis Spiny Aster Baccharis, Clematis √ Oct. 29, 2012 Gray Hairstreak - Strymon melinus Frostweed, Aster Many flowering plants √ Oct. 29, 2012 Great Purple Hairstreak - Atlides halesus Spiny Aster Mistletoe (in oak/mesquite) √ Oct. 29, 2012 Marine Blue - Leptotes marina Frogfruit Legumes- Acacia, Mesquite √ Oct. 30, 2012 Family Nymphalidae (Nymphalids) American Snout - Libytheana carinenta Spiny Aster Hackberries √ Oct. 29, 2012 Bordered Patch - Chlosyne lacinia Zexmenia Ragweed, Sunflower √ Oct. 29, 2012 Common Mestra - Mestra amymone Frostweed Noseburn (Tragia spp.) X Oct. 29, 2012 Empress Leila - Asterocampa leilia Spiny Aster Spiny Hackberry √ Oct. 29, 2012 Gulf Fritillary - Agraulis vanillae Spiny Aster Passion Vine X Oct. 29, 2012 Hackberry Emperor - Asterocampa celtis spiny Hackberry Hackberries √ Oct. 29, 2012 Painted Lady - Vanessa cardui Pink Smartweed Mallows & Thistles √ Oct. 29, 2012 Pearl Crescent - Phycoides tharos Spiny Aster Asters √ Oct. -

Butterflies of Tennessee Alphabetical by Common Name Butterflies Of
1 Butterflies of Tennessee Butterflies of Tennessee Alphabetical by Common Name Page 2 Butterflies of Tennessee Alphabetical by Scientific Name Page 6 Butterflies of Tennessee Alphabetical by Family Page 10 The Middle Tennessee Chapter of the North American Butterfly Association (NABA) maintains the list of Butterflies in Tennessee. Check their website at: nabamidtn.org/?page_id=176 Updated March 2015 1 2 Butterflies of Tennessee Alphabetical by Common Name Common Name Scientific Name Family American Copper Lycaena phlaeas Lycaenidae American Lady Vanessa virginiensis Nymphalidae American Snout Libytheana carinenta Nymphalidae Aphrodite Fritillary Speyeria aphrodite Nymphalidae Appalachian Azure Celestrina neglectamajor Lycaenidae Appalachian Brown Satyrodes appalachia Nymphalidae Appalachian Tiger Swallowtail Papilio appalachiensis Papilionidae Baltimore Checkerspot Euphydryas phaeton Nymphalidae Banded Hairstreak Satyrium calanus Lycaenidae Bell’s Roadside-Skipper Amblyscirtes belli Hesperiidae Black Swallowtail Papilio polyxenes Papilionidae Brazilian Skipper Calpodes ethlius Hesperiidae Broad-winged Skipper Poanes viator Hesperiidae Bronze Copper Lycaena hyllus Lycaenidae Brown Elfin Callophrys augustinus Lycaenidae Cabbage White Pieris rapae Pieridae Carolina Satyr Hermeuptychia sosybius Nymphalidae Checkered White Pontia protodice Pieridae Clouded Skipper Lerema accius Hesperiidae Clouded Sulphur Colias philodice Pieridae Cloudless Sulphur Phoebis sennae Pieridae Cobweb Skipper Hesperia metea Hesperiidae Common Buckeye Junonia coenia -

Conservation of the Arogos Skipper, Atrytone Arogos Arogos (Lepidoptera: Hesperiidae) in Florida Marc C
Conservation of the Arogos Skipper, Atrytone arogos arogos (Lepidoptera: Hesperiidae) in Florida Marc C. Minno St. Johns River Water Management District P.O. Box 1429, Palatka, FL 32177 [email protected] Maria Minno Eco-Cognizant, Inc., 600 NW 35th Terrace, Gainesville, FL 32607 [email protected] ABSTRACT The Arogos skipper is a rare and declining butterfly found in native grassland habitats in the eastern and mid- western United States. Five distinct populations of the butterfly occur in specific parts of the range. Atrytone arogos arogos once occurred from southern South Carolina through eastern Georgia and peninsular Florida as far south as Miami. This butterfly is currently thought to be extirpated from South Carolina and Georgia. The six known sites in Florida for A. arogos arogos are public lands with dry prairie or longleaf pine savanna having an abundance of the larval host grass, Sorghastrum secundum. Colonies of the butterfly are threat- ened by catastrophic events such as wild fires, land management activities or no management, and the loss of genetic integrity. The dry prairie preserves of central Florida will be especially important to the recovery of the butterfly, since these are some of the largest and last remaining grasslands in the state. It may be possible to create new colonies of the Arogos skipper by releasing wild-caught females or captive-bred individuals into currently unoccupied areas of high quality habitat. INTRODUCTION tered colonies were found in New Jersey, North Carolina, South Carolina, Florida, and Mississippi. The three re- gions where the butterfly was most abundant included The Arogos skipper (Atrytone arogos) is a very locally the New Jersey pine barrens, peninsular Florida, and distributed butterfly that occurs only in the eastern and southeastern Mississippi. -

How to Use This Checklist
How To Use This Checklist Swallowtails: Family Papilionidae Special Note: Spring and Summer Azures have recently The information presented in this checklist reflects our __ Pipevine Swallowtail Battus philenor R; May - Sep. been recognized as separate species. Azure taxonomy has not current understanding of the butterflies found within __ Zebra Swallowtail Eurytides marcellus R; May - Aug. been completely sorted out by the experts. Cleveland Metroparks. (This list includes all species that have __ Black Swallowtail Papilio polyxenes C; May - Sep. __ Appalachian Azure Celastrina neglecta-major h; mid - late been recorded in Cuyahoga County, and a few additional __ Giant Swallowtail Papilio cresphontes h; rare in Cleveland May; not recorded in Cuy. Co. species that may occur here.) Record you observations and area; July - Aug. Brush-footed Butterflies: Family Nymphalidae contact a naturalist if you find something that may be of __ Eastern Tiger Swallowtail Papilio glaucus C; May - Oct.; __ American Snout Libytheana carinenta R; June - Oct. interest. females occur as yellow or dark morphs __ Variegated Fritillary Euptoieta claudia R; June - Oct. __ Spicebush Swallowtail Papilio troilus C; May - Oct. __ Great Spangled Fritillary Speyeria cybele C; May - Oct. Species are listed taxonomically, with a common name, a Whites and Sulphurs: Family Pieridae __ Aphrodite Fritillary Speyeria aphrodite O; June - Sep. scientific name, a note about its relative abundance and flight __ Checkered White Pontia protodice h; rare in Cleveland area; __ Regal Fritillary Speyeria idalia X; no recent Ohio records; period. Check off species that you identify within Cleveland May - Oct. formerly in Cleveland Metroparks Metroparks. __ West Virginia White Pieris virginiensis O; late Apr. -

American Snout,Libytheana Carinenta (Cramer)
EENY 452 American snout, Libytheana carinenta (Cramer) (Insecta: Lepidoptera: Nymphalidae: Libytheinae)1 Donald W. Hall and Jerry F. Butler2 Introduction Synonomy The American snout, Libytheana carinenta (Cramer), is a Libytheana bachmanii (Kirtland) is a synonym of Libythe- small, relatively dull colored butterfly that is named because ana carinenta (Cramer) (Heppner 2003). of the pronounced elongation of its labial palpi into a prominent snout. The specific epithet, carinenta, from the Distribution Latin root “carin” presumably refers to the keel-shape of the The American snout is found from southern and central snout. California northeastward to Colorado and Nebraska and throughout most of the eastern U.S. It is common in northern and central Florida, but is infrequent in southern Florida. It also occurs through Central America and South America to Argentina (Minno et al. 2005, Opler et al. 2009). Description Adults The wing spread of adults is 1.6 to 1.9 inches (Daniels 2003). The upper surface of the wings is blackish-brown with orange patches on the basal half of both fore and hind wings and white spots on the distal half of the forewings. The tip of the forewing is squared. The underside of the fore wings is similar to the upper side except the marginal areas may be lighter. The underside of the hind wings may be mottled or smooth purplish gray or brown. Figure 1. Dorsal view of the wings of an adult American snout, Libytheana carinenta (Cramer). (Hendricks County, Indiana; June 22, There are a number of regional varieties that were once 2001). considered to be different species but are now considered to Credits: Don Hall, University of Florida be subspecies of Libytheana carinenta. -

Status and Trends of Wild Insect Pollinators in Vermont and Beyond
Status and trends of wild insect pollinators in Vermont and beyond Mark Ferguson Vermont Fish and Wildlife Department Kent McFarland Vermont Center for Ecostudies Leif Richardson Gund Institute for Ecological Economics, UVM What is the status of pollinators? • Widespread reports of decline—and persistence • Loss of ecosystem function? • Loss of ecosystem service to agriculture? • How do we know? What do we mean by pollinator ‘declines’? • Reduction in overall pollinator density/ abundance • Reduction in species diversity or shifts in community • Consequent decline in plant reproductive success ‘Case studies’ for 3 insect pollinators • Flies, Lepidoptera and bees • Status in Vermont and beyond, if known • Examples 1. Flies (Diptera) • 71 fly families feed at flowers • 15,000+ species in North America • Pollinate >100 crop plants, including mango, onion, canola, cacao • Changes in hoverfly diversity in UK, Netherlands • What about Vermont? Vermont fly pollinators 2. Butterflies and moths (Lepidoptera) • 700+/ 13,000+ species in North America • Declines of VT grassland butterflies • Declines, range retractions and shifts: California, US prairies, UK, EU • Vermont? Vermont Lepidoptera pollinators 3. Bees (Hymenoptera) • 4,000+ species in North America; ~275 in Vermont • Plant pollen = protein source for bees • Range retractions: EU, UK, North America • Widespread declines in diversity (and abundance?) • Evidence of competition with non-native bees driving some declines Bee declines • In eastern North America, bumble bees in decline, but most -

1 Modern Threats to the Lepidoptera Fauna in The
MODERN THREATS TO THE LEPIDOPTERA FAUNA IN THE FLORIDA ECOSYSTEM By THOMSON PARIS A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2011 1 2011 Thomson Paris 2 To my mother and father who helped foster my love for butterflies 3 ACKNOWLEDGMENTS First, I thank my family who have provided advice, support, and encouragement throughout this project. I especially thank my sister and brother for helping to feed and label larvae throughout the summer. Second, I thank Hillary Burgess and Fairchild Tropical Gardens, Dr. Jonathan Crane and the University of Florida Tropical Research and Education center Homestead, FL, Elizabeth Golden and Bill Baggs Cape Florida State Park, Leroy Rogers and South Florida Water Management, Marshall and Keith at Mack’s Fish Camp, Susan Casey and Casey’s Corner Nursery, and Michael and EWM Realtors Inc. for giving me access to collect larvae on their land and for their advice and assistance. Third, I thank Ryan Fessendon and Lary Reeves for helping to locate sites to collect larvae and for assisting me to collect larvae. I thank Dr. Marc Minno, Dr. Roxanne Connely, Dr. Charles Covell, Dr. Jaret Daniels for sharing their knowledge, advice, and ideas concerning this project. Fourth, I thank my committee, which included Drs. Thomas Emmel and James Nation, who provided guidance and encouragement throughout my project. Finally, I am grateful to the Chair of my committee and my major advisor, Dr. Andrei Sourakov, for his invaluable counsel, and for serving as a model of excellence of what it means to be a scientist. -

Exploring Tradeoffs of Alternative Life History Strategies In
EXPLORING TRADEOFFS OF ALTERNATIVE LIFE HISTORY STRATEGIES IN TWO TEXAS BUTTERFLY SPECIES by Amara Lee Garza B.S. A thesis submitted to the Graduate Council of Texas State University in partial fulfillment of the requirements for the degree of Master of Science with a Major in Population and Conservation Biology May 2018 Committee Members: Chris C. Nice, Chair Jim R. Ott Susan Schwinning COPYRIGHT by Amara Lee Garza 2018 FAIR USE AND AUTHOR’S PERMISSION STATEMENT Fair Use This work is protected by the Copyright Laws of the United States (Public Law 94-553, section 107). Consistent with fair use as defined in the Copyright Laws, brief quotations from this material are allowed with proper acknowledgement. Use of this material for financial gain without the author’s express written permission is not allowed. Duplication Permission As the copyright holder of this work I, Amara Lee Garza refuse permission to copy in excess of the “Fair Use” exemption without my written permission. DEDICATION I would like to dedicate this work to all the future Latinx scientists, and to my four siblings Avana Bree, Abram Three, Amaya Cree and Alain Zee. ACKNOWLEDGEMENTS I would like to thank my committee members Dr. Nice, Dr. Ott and Dr. Schwinning for their advice and support. I would also like to thank my mom Gabriela Quintero for her support, encouragement, and unfading faith in me. v TABLE OF CONTENTS Page ACKNOWLEDGEMENTS……………………………………………………………. v LIST OF FIGURES……………………………………………………………………. vii ABSTRACT…………………………………………………………………………… viii CHAPTER I. EXPLORING -

John Abbot's Butterfly Drawings for William Swainson
VOLUME 61, NUMBER 1 1 J OURNAL OF T HE L EPIDOPTERISTS’ S OCIETY Volume 61 2007 Number 1 Journal of the Lepidopterists’ Society 61(1), 2007, 1–20 JOHN ABBOT’S BUTTERFLY DRAWINGS FOR WILLIAM SWAINSON, INCLUDING GENERAL COMMENTS ABOUT ABBOT’S ARTISTIC METHODS AND WRITTEN OBSERVATIONS JOHN V. C ALHOUN1 977 Wicks Dr., Palm Harbor, FL 34684 ABSTRACT. Between 1816 and 1818, artist-naturalist John Abbot completed 103 drawings of insects for English naturalist William Swain- son. The history of these illustrations is reviewed, leading up to their rediscovery in 1977 in the Alexander Turnbull Library, Wellington, New Zealand. Four of these drawings are figured. The adults in the 21 butterfly drawings are identified and the figures of larvae and pupae are as- sessed for accuracy. The illustrated plants are also identified and their status as hosts is examined. Aspects of Abbot’s life history notes are dis- cussed, including his spelling, grammar, and use of Latin names. His notes for Swainson are transcribed and analyzed. A review of Abbot’s art- work indicates that he duplicated many of his compositions for 20–25 years. He sometimes portrayed erroneous figures of larvae, pupae, and hostplants. Figures of immatures were sometimes fabricated using other species as models. He also applied duplicate figures of larvae to more than one species. Abbot may have sent another set of insect drawings to Swainson in 1830. Ninety-nine smaller drawings at the Turnbull Li- brary are attributed to both Abbot and Swainson. Six of these illustrations are figured. Abbot’s notes for Swainson suggest that at least three butterfly species are now more abundant than during the early nineteenth century, while three others are probably less widespread than for- merly. -

An Annotated Checklist of the Butterflies of Athens County, Ohio1
262 P. A. FROELICH AND L. A. MESERVE Vol. 83 Copyright © 1983 Ohio Acad. Sci. 0030-0950/83/0005-0262 $2.00/0 AN ANNOTATED CHECKLIST OF THE BUTTERFLIES OF ATHENS COUNTY, OHIO1 JOHN A. SHUEY, Department of Entomology, The Ohio State University, Columbus, OH 43210 ABSTRACT. Ninety-two species of butterflies are reported from Athens County, Ohio; 81 of these species for the first time. The following information is recorded for each species: abundance, habitat, collection localities, and extreme dates of capture. Eleven of these species have not been reported previously from any counties adjacent to the study area. These species are Stapbylus hayhurstii, Thymelkus lineola, Hylephila phyleus, Papilio cres- phontes, Colias cesonia, Pboebis sennae, Nathalis iole, Calephelis borealis, Ltbytheana bachmanii, Asterocampa clyton, and Euptychia sosybius. A listing of 8 additional species which might be expected to occur in the county is also included. OHIO J. SCI. 83 (5): 262-269, 1983 INTRODUCTION lished (Porter 1965, Studebaker and Athens County has one of the least Studebaker 1967, Price 1970, Albrecht known butterfly faunas in Ohio. Although ^74, Drees 1982), all of them deal with several recent faunal lists have been pub- western Ohio. Many older lists have been published, but prior to Lindsey's (1921) 'Manuscript received 10 December 1982 and in work, determinations of Hesperiidae were revised form 19 April 1983 (#82-39). very unreliable (Bales 1909). Albrecht's OhioJ. Sci. BUTTERFLIES OF ATHENS COUNTY 263 (1982) dissertation provides much infor- 1969). The original vegetation consisted of mation about Ohio's fauna, but he records beech-maple forest in mesic areas and only 10 species from Athens County.