Exploring Tradeoffs of Alternative Life History Strategies In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Celtis Tenuifolia) in Ontario
Photo: Allen Woodliffe Dwarf Hackberry (Celtis tenuifolia) in Ontario Ontario Recovery Strategy Series Recovery strategy prepared under the Endangered Species Act, 2007 2013 Ministry of Natural Resources About the Ontario Recovery Strategy Series This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act (ESA) and the Accord for the Protection of Species at Risk in Canada. What is recovery? What’s next? Recovery of species at risk is the process by which the Nine months after the completion of a recovery strategy decline of an endangered, threatened, or extirpated a government response statement will be published species is arrested or reversed, and threats are which summarizes the actions that the Government of removed or reduced to improve the likelihood of a Ontario intends to take in response to the strategy. species’ persistence in the wild. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and What is a recovery strategy? conservationists. Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to For more information achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the To learn more about species at risk recovery in Ontario, survival and recovery of the species. It also makes please visit the Ministry of Natural Resources Species at recommendations on the objectives for protection and Risk webpage at: www.ontario.ca/speciesatrisk recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. -

Caterpillars Moths Butterflies Woodies
NATIVE Caterpillars Moths and utter flies Band host NATIVE Hackberry Emperor oodies PHOTO : Megan McCarty W Double-toothed Prominent Honey locust Moth caterpillar Hackberry Emperor larva PHOTO : Douglas Tallamy Big Poplar Sphinx Number of species of Caterpillars n a study published in 2009, Dr. Oaks (Quercus) 557 Beeches (Fagus) 127 Honey-locusts (Gleditsia) 46 Magnolias (Magnolia) 21 Double-toothed Prominent ( Nerice IDouglas W. Tallamy, Ph.D, chair of the Cherries (Prunus) 456 Serviceberry (Amelanchier) 124 New Jersey Tea (Ceanothus) 45 Buttonbush (Cephalanthus) 19 bidentata ) larvae feed exclusively on elms Department of Entomology and Wildlife Willows (Salix) 455 Larches or Tamaracks (Larix) 121 Sycamores (Platanus) 45 Redbuds (Cercis) 19 (Ulmus), and can be found June through Ecology at the University of Delaware Birches (Betula) 411 Dogwoods (Cornus) 118 Huckleberry (Gaylussacia) 44 Green-briar (Smilax) 19 October. Their body shape mimics the specifically addressed the usefulness of Poplars (Populus) 367 Firs (Abies) 117 Hackberry (Celtis) 43 Wisterias (Wisteria) 19 toothed shape of American elm, making native woodies as host plants for our Crabapples (Malus) 308 Bayberries (Myrica) 108 Junipers (Juniperus) 42 Redbay (native) (Persea) 18 them hard to spot. The adult moth is native caterpillars (and obviously Maples (Acer) 297 Viburnums (Viburnum) 104 Elders (Sambucus) 42 Bearberry (Arctostaphylos) 17 small with a wingspan of 3-4 cm. therefore moths and butterflies). Blueberries (Vaccinium) 294 Currants (Ribes) 99 Ninebark (Physocarpus) 41 Bald cypresses (Taxodium) 16 We present here a partial list, and the Alders (Alnus) 255 Hop Hornbeam (Ostrya) 94 Lilacs (Syringa) 40 Leatherleaf (Chamaedaphne) 15 Honey locust caterpillar feeds on honey number of Lepidopteran species that rely Hickories (Carya) 235 Hemlocks (Tsuga) 92 Hollies (Ilex) 39 Poison Ivy (Toxicodendron) 15 locust, and Kentucky coffee trees. -

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

The Plant Press the ARIZONA NATIVE PLANT SOCIETY
The Plant Press THE ARIZONA NATIVE PLANT SOCIETY Volume 36, Number 1 Summer 2013 In this Issue: Plants of the Madrean Archipelago 1-4 Floras in the Madrean Archipelago Conference 5-8 Abstracts of Botanical Papers Presented in the Madrean Archipelago Conference Southwest Coralbean (Erythrina flabelliformis). Plus 11-19 Conservation Priority Floras in the Madrean Archipelago Setting for Arizona G1 Conference and G2 Plant Species: A Regional Assessment by Thomas R. Van Devender1. Photos courtesy the author. & Our Regular Features Today the term ‘bioblitz’ is popular, meaning an intensive effort in a short period to document the diversity of animals and plants in an area. The first bioblitz in the southwestern 2 President’s Note United States was the 1848-1855 survey of the new boundary between the United States and Mexico after the Treaty of Guadalupe Hidalgo of 1848 ended the Mexican-American War. 8 Who’s Who at AZNPS The border between El Paso, Texas and the Colorado River in Arizona was surveyed in 1855- 9 & 17 Book Reviews 1856, following the Gadsden Purchase in 1853. Besides surveying and marking the border with monuments, these were expeditions that made extensive animal and plant collections, 10 Spotlight on a Native often by U.S. Army physicians. Botanists John M. Bigelow (Charphochaete bigelovii), Charles Plant C. Parry (Agave parryi), Arthur C. V. Schott (Stephanomeria schotti), Edmund K. Smith (Rhamnus smithii), George Thurber (Stenocereus thurberi), and Charles Wright (Cheilanthes wrightii) made the first systematic plant collection in the Arizona-Sonora borderlands. ©2013 Arizona Native Plant In 1892-94, Edgar A. Mearns collected 30,000 animal and plant specimens on the second Society. -

Climate Ready Trees for Central Valley Communities
The goal of this study is to evaluate the survival and growth of seldom used but promising trees in the Central Valley. We can create more resilient Climate Ready Trees for urban forests by shifting the palate of trees planted to those proven to Central Valley Communities perform best when exposed to climate stressors such as heat, drought, high winds, pests, disease and soil salinity. 1. Mulga (Acacia aneura) Mulga is native to arid Western Australia and tolerates hot and dry conditions. It can grow in sandy, loam, or clay soil types. This versatile and hardy tree produces ascending thornless branches and grows 15 to 20 feet in height. The evergreen foliage is gray-green and the tree has yellow flowers in the spring. Maintain leader to avoid suckering. 2. Netleaf Hackberry (Celtis reticulata) The Netleaf Hackberry is native to riparian areas in the Southwest. A deciduous tree, it reaches heights of 25 to 35 feet with a spreading or weeping canopy. The ovate leaves are medium green and turn yellow in the fall. The flowers mature into red drupes that attract birds. The Netleaf Hackberry is drought tolerant and able to thrive in variety of soil types. 3. Desert Willow (Chilopsis linearis ‘Bubba’) The Desert Willow is a small flowering desert tree native to California and the Southwest. The cultivar Bubba can reach 25 to 30 feet with a spread of 20 to 25 feet. It has profuse, long- lasting blooms. The showy flowers are pink and white. Leaves are linear blue green and turn golden in the fall. -

Macfarlane's Four O'clock (Mirabilis Macfarlanei)
Macfarlane's four o'clock (Mirabilis macfarlanei) ENDANGERED Flowers (left), habit (center), and habitat (right) of Macfarlane’s four o’clock. Photos by Thomas Kaye. If downloading images from this website, please credit the photographer. Family Nyctaginaceae Plant description Macfarlane’s four o’clock is a stout perennial that forms hemispheric clumps 0.6-1.2 m in diameter, with several freely branched decumbent or ascending stems that are glabrous to sparsely puberulent. The leaves are opposite and fleshy, the lower blades orbicular to ovate-deltoid, the upper narrowly ovate. The petioles of lower leaves are 1- 2.5 cm long; upper leaves are nearly sessile. Flowers are clustered (4-7) in involucres borne on stalks about 1 cm long in the upper axils and on shoot apices. The conspicuous involucres are green to purplish, 1.3-2.5 cm long. The showy perianth is magenta, broadly funnelform, and 1.5-2.5 cm long, the limb slightly longer than the tube. The ellipsoid fruits are light brown to grayish, with 10 slender ribs visible when wet, 6-9 mm long, tuberculate, glabrous or very sparsely puberulent. Distinguishing characteristics No other Mirabilis species occur within the range of Macfarlane’s four o’clock. Mirabilis laevis var. retrorsa has shorter involucres (0.5-0.7 cm long), a white to pale pink perianth, and ranges from Malheur and Harney Counties in Oregon southward. Macfarlane’s four o’clock is most closely related to M. multiflora var. glandulosa and M. greenei, which occur in Nevada and California, respectively, and have longer involucres and larger perianths. -

Lepidoptera, Nymphalidae, Biblidinae) and Patterns of Morphological Similarity Among Species from Eight Tribes of Nymphalidae
Revista Brasileira de Entomologia http://dx.doi.org/10.1590/S0085-56262013005000006 External morphology of the adult of Dynamine postverta (Cramer) (Lepidoptera, Nymphalidae, Biblidinae) and patterns of morphological similarity among species from eight tribes of Nymphalidae Luis Anderson Ribeiro Leite1,2, Mirna Martins Casagrande1,3 & Olaf Hermann Hendrik Mielke1,4 1Departamento de Zoologia, Setor de Ciências Biológicas, Universidade Federal do Paraná, Caixa Postal 19020, 81531–980 Curitiba-PR, Brasil. [email protected], [email protected], [email protected] ABSTRACT. External morphology of the adult of Dynamine postverta (Cramer) (Lepidoptera, Nymphalidae, Biblidinae) and patterns of morphological similarity among species from eight tribes of Nymphalidae. The external structure of the integument of Dynamine postverta postverta (Cramer, 1779) is based on detailed morphological drawings and scanning electron microscopy. The data are compared with other species belonging to eight tribes of Nymphalidae, to assist future studies on the taxonomy and systematics of Neotropical Biblidinae. KEYWORDS. Abdomen; head; Insecta; morphology; Papilionoidea; thorax. Nymphalidae is a large cosmopolitan family of butter- served in dorsal view (Figs. 1–4). Two subspecies are recog- flies, with about 7,200 described species (Freitas & Brown nized according to Lamas (2004), Dynamine postverta Jr. 2004) and is perhaps the most well documented biologi- postverta (Cramer, 1779) distributed in South America and cally (Harvey 1991; Freitas & Brown Jr. 2004; Wahlberg et Dynamine postverta mexicana d’Almeida, 1952 with a dis- al. 2005). The systematic relationships are still somewhat tribution restricted to Central America. Several species sur- unclear with respect to its subfamilies, tribes and genera, and veys and other studies cite this species as Dynamine mylitta even after more than a century of studies on these groups, (DeVries 1987; Mielke 1994; Miller et al.1999; Freitas & these relationships still seem to confuse many who set out to Brown, Jr. -

Arthropods of Elm Fork Preserve
Arthropods of Elm Fork Preserve Arthropods are characterized by having jointed limbs and exoskeletons. They include a diverse assortment of creatures: Insects, spiders, crustaceans (crayfish, crabs, pill bugs), centipedes and millipedes among others. Column Headings Scientific Name: The phenomenal diversity of arthropods, creates numerous difficulties in the determination of species. Positive identification is often achieved only by specialists using obscure monographs to ‘key out’ a species by examining microscopic differences in anatomy. For our purposes in this survey of the fauna, classification at a lower level of resolution still yields valuable information. For instance, knowing that ant lions belong to the Family, Myrmeleontidae, allows us to quickly look them up on the Internet and be confident we are not being fooled by a common name that may also apply to some other, unrelated something. With the Family name firmly in hand, we may explore the natural history of ant lions without needing to know exactly which species we are viewing. In some instances identification is only readily available at an even higher ranking such as Class. Millipedes are in the Class Diplopoda. There are many Orders (O) of millipedes and they are not easily differentiated so this entry is best left at the rank of Class. A great deal of taxonomic reorganization has been occurring lately with advances in DNA analysis pointing out underlying connections and differences that were previously unrealized. For this reason, all other rankings aside from Family, Genus and Species have been omitted from the interior of the tables since many of these ranks are in a state of flux. -
The Butterflies of Mississippi Supplement No
Journal of the Lepidopterists' Society 39(2), 1985, 134-138 THE BUTTERFLIES OF MISSISSIPPI SUPPLEMENT NO. 31 BRYANT MATHER2 AND KATHARINE MATHER 213 Mt. Salus Road, Clinton, Mississippi 39056 ABSTRACT. An annotated list of Mississippi butterflies is presented. This updated version is the fifth such list published. Six names additional to the previous lists have been included. Of the five published lists of Mississippi butterflies, this is the first to use the names and arrangement of Miller and Brown (1981) as amend ed by them in Hodges (Editor) (1983). It includes six names not in cluded in the fourth list (Mather & Mather, 1976). The growth rate has dropped to fewer than one per year as indicated in Table 1 below: TABLE 1. Published lists of Mississippi butterflies, showing rate of increase in the addition of names previously unrecorded from the state. Time Names added List Reference Names interval Names added per year 1 Weed (1894) 53 2 Hutchins (1933) 73 39 20 0.5 3 M. & M. (1958) 122 25 49 1.9 4 M. & M. (1976) 144 18 22 1.2 5 M. & M. (1984) 150 8 6 0.75 In 1958 we expressed the opinion that the list would grow to include about 160 names. We also said, "there may be cases in which the Mississippi representatives of a given species represent more than one population; if so, we do not believe that we as yet have adequate data to support such a conclusion." Now we do. Our reasons for adding the six names are summarized below. 1. -
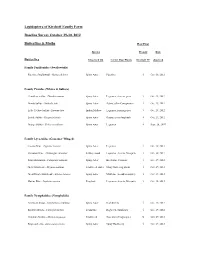
Butterflies and Moths List
Lepidoptera of Kirchoff Family Farm Baseline Survey October 29-30, 2012 Butterflies & Moths Host Plant Species Present Date Butterflies Observed On Larval Host Plants Kirchoff FF observed Family Papilionidae (Swallowtails) Pipevine Swallowtail - Battus philenor Spiny Aster Pipevine √ Oct. 30, 2012 Family Pieridae (Whites & Sulfurs) Cloudless Sulfur - Phoebis sennae Spiny Aster Legumes, clovers, peas √ Oct. 29, 2012 Dainty Sulfur - Nathalis iole Spiny Aster Asters, other Com;posites √ Oct. 29, 2012 Little Yellow Sulfur - Eurema lisa Indian Mallow Legumes, partridge pea √ Oct. 29, 2012 Lyside Sulfur - Kirgonia lyside Spiny Aster Guayacan or Soapbush √ Oct. 29, 2012 Orange Sulfur - Colias eurytheme Spiny Aster Legumes √ Sept. 26, 2007 Family Lycaenidae (Gossamer Winged) Cassius Blue - Leptotes cassius Spiny Aster Legumes √ Oct. 30, 2012 Ceraunus Blue - Hemiargus ceraunus Kidney wood Legumes- Acacia, Mesquite √ Oct. 30, 2012 Fatal Metalmark - Calephelis nemesis Spiny Aster Baccharis, Clematis √ Oct. 29, 2012 Gray Hairstreak - Strymon melinus Frostweed, Aster Many flowering plants √ Oct. 29, 2012 Great Purple Hairstreak - Atlides halesus Spiny Aster Mistletoe (in oak/mesquite) √ Oct. 29, 2012 Marine Blue - Leptotes marina Frogfruit Legumes- Acacia, Mesquite √ Oct. 30, 2012 Family Nymphalidae (Nymphalids) American Snout - Libytheana carinenta Spiny Aster Hackberries √ Oct. 29, 2012 Bordered Patch - Chlosyne lacinia Zexmenia Ragweed, Sunflower √ Oct. 29, 2012 Common Mestra - Mestra amymone Frostweed Noseburn (Tragia spp.) X Oct. 29, 2012 Empress Leila - Asterocampa leilia Spiny Aster Spiny Hackberry √ Oct. 29, 2012 Gulf Fritillary - Agraulis vanillae Spiny Aster Passion Vine X Oct. 29, 2012 Hackberry Emperor - Asterocampa celtis spiny Hackberry Hackberries √ Oct. 29, 2012 Painted Lady - Vanessa cardui Pink Smartweed Mallows & Thistles √ Oct. 29, 2012 Pearl Crescent - Phycoides tharos Spiny Aster Asters √ Oct. -

Lepidoptera: Erebidae: Arctiinae) Filled with Crystallizing Material
Journal of Insect Science, (2019) 19(5): 21; 1–12 doi: 10.1093/jisesa/iez099 Research ‘Crystal Macrosetae’: Novel Scales and Bristles in Male Arctiine Moths (Lepidoptera: Erebidae: Arctiinae) Filled with Crystallizing Material Michael Boppré,1, Ottmar W. Fischer, Hannes Freitag, and Anita Kiesel Forstzoologie und Entomologie, Albert-Ludwigs-Universität, D-79085 Freiburg i.Br., Germany and 1Corresponding author, e-mail: [email protected] Subject Editor: Phyllis Weintraub Received 9 August 2019; Editorial decision 5 September 2019 Abstract Scales, exoskeletal features characteristic of the Lepidoptera, occur in enormous structural and functional diversity. They cover the wing membranes and other body parts and give butterflies and moths their often stunning appearance. Generally, the patterns made by scales are visual signals for intra- and interspecific communication. In males, scales and/or bristles also make up the androconial organs, which emit volatile signals during courtship. Here, a structurally and putative functionally novel type of scales and bristles is reported: ‘crystal macrosetae’. These lack trabeculae and windows, are made up by a very thin and flexible envelope only and contain crystallizing material. In ‘crystal scales’, there is a flat surface ornamentation of modified ridges, while ‘crystal bristles’ often show large protrusions. Crystal macrosetae usually cannot be reliably recognized without destruction. Apparently, they serve as containers for large amounts of material that is viscous in living moths, highly hygroscopic, crystallizes when specimens dry up, and can be visualized by scanning electron microscopy. Crystal macrosetae occur in males only, always associated with or making up androconial organs located on various parts of the body, and have numerous forms with diverse surface ornamentation across many species and genera. -

Hymenoptera: Braconidae) Reared from Hypercompe Cunigunda (Lepidoptera: Erebidae) in Brazil
Revista Brasileira de Entomologia 64(1):e201982, 2020 www.rbentomologia.com Diolcogaster choi sp. nov. from Brazil, a new gregarious microgastrine parasitoid wasp (Hymenoptera: Braconidae) reared from Hypercompe cunigunda (Lepidoptera: Erebidae) in Brazil Geraldo Salgado-Neto1* , Ísis Meri Medri2, José L. Fernández-Triana3, James Bryan Whitfield4 1Universidade Federal de Santa Maria, Departamento de Defesa Fitossanitária, Pós-graduação em Agronomia, Santa Maria, RS, Brasil. 2Universidade de Brasília, Departamento de Ecologia, Doutorado em Ecologia, Brasília, D F, Brasil. 3Canadian National Collection of Insects, Arachnids, and Nematodes, Ottawa, Ontario, Canada. 4University of Illinois at Urbana-Champaign, Department of Entomology, Urbana, USA. urn:lsid:zoobank.org:pub:28F860D2-5CDB-4D55-82BC-C41CFE1ADD0E ARTICLE INFO ABSTRACT Article history: A new species of Diolcogaster (Hymenoptera: Braconidae) is described and illustrated. Additionally, its position Received 23 August 2019 within the recently published key to New World species of the xanthaspis species-group (to which the described Accepted 17 December 2019 Diolcogaster belongs) is provided. The gregarious larval parasitoid Diolcogaster choi sp. nov. was collected in Available online 17 February 2020 Maringá, Paraná State, Brazil. This natural enemy was recovered from a caterpillar of Hypercompe cunigunda (Stoll, Associate Editor: Bernardo Santos 1781) (Lepidoptera: Erebidae) that was feeding on plant of passionflower, Passiflora edulis Sims (Passifloraceae). The fauna of the xanthaspis group in the New World now includes five species, including the new species from Brazil described in this paper. Diolcogaster choi sp. nov. differs anatomically, and is morphologically diagnosed, Keywords: from all other known member of the xanthaspis group of the genus Diolcogaster, to which it belongs. The species Caterpillar also differs in recorded host, and its DNA barcode appears to be distinctive among described Diolcogaster.