Radical Transformation of the Wing Pattern in Asterocampa Clyton Caused by Heparin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Butterflies of the Finger Lakes National Forest
The Finger Lakes National Butterflies of "... the first precaution Forest comprises 16,000 acres of the of intelligent tinkering federally owned land between Cayuga and Seneca Lakes in Finger Lakes is to keep every cog and New York State. The Forest is wheel ... " managed for multiple uses to ~~Mut provide a variety of goods and ~Aldo Leopold services to a diverse public. Different programs include a This list compiled through the work of livestock grazing program, a Charles R. Smith, Ph.D. Department of Natural Resources small scale timber management Cornell University program, diverse habitats for And Donald Bright-Smith, Ph.D. wildlife and fish, and Department of Pathobiology recreational opportunities for TexasA&M multiple user groups. And made possible through the generosity New York State Chapter of the For more information about the Finger Lakes Wild Turkey Federation National Forest contact: Hector Ranger District 5218 State Route 414 United States Forest Eastern ~~1~ .. ~ Department of Service Region New York State Chapter Hector, NY 14841 Agriculture NWTF (607) 546-4470 Family Papilionidae: Swallowtails Family Hesperiidae: Skippers 0 Papilio polyxenes, Black Swallowtail 0 Epargyreus clarus, Silver-spotted 0 Pap ilia cresphontes, Giant Swallowtail Skipper 0 Papilio glaucus, Eastern Tiger Family Nymphalidae: Brushfoots 0 Erynnis ice/us, Dreamy Duskywing Swallowtail 0 Speyeria cybele, Great Spangled Fritillary D Erynnisjuvenalis, Juvenal's Duskywing 0 Pap ilia troilus, Spicebush Swallowtail 0 Boloria selene, Silver-bordered Fritillary -

Butterfly Gardening Tips & Tricks Gardening for Butterflies Is Fun, Beautiful, and Good for the Environment
Butterfly Gardening Tips & Tricks Gardening for butterflies is fun, beautiful, and good for the environment. It is also simple and can be done in almost any location. The key guidelines are listed below: NO PESTICIDES! Caterpillars are highly susceptible to almost all pesticides so keep them away from your yard if you want butterflies to thrive. Select the right plants. You will need to provide nectar sources for adults and host plants for caterpillars. See the lists below for inspiration. Keep to native varieties as much as possible. Plants come in lots and lots of varieties and cultivars. When selecting plants, especially host plants, try to find native species as close to the natural or wild variety as possible. Provide shelter. Caterpillars need shelter from the sun and shelter from cold nights. Adults need places to roost during the night. And protected areas are needed for the chrysalis to safely undergo its transformation. The best way to provide shelter is with large clumps of tall grasses (native or ornamental) and medium to large evergreen trees and/or shrubs. Nectar Sources Top Ten Nectar Sources: Asclepias spp. (milkweed) Aster spp. Buddleia spp. (butterfly bush) Coreopsis spp. Echinacea spp. (coneflower) Eupatorium spp. (joe-pye weed) Lantana spp. Liatris spp. Pentas spp. Rudbeckia spp. (black-eyed susan) Others: Agastache spp. (hyssop), Apocynum spp. (dogbane), Ceanothus americanus (New Jersey tea), Cephalanthus occidentalis (button bush), Clethra alnifolia, Cuphea spp. (heather), Malus spp. (apple), Mentha spp. (mint), Phlox spp., Pycanthemum incanum (mountain mint), Salivs spp. (sage), Sedum spectabile (stonecrop), Stokesia laevis (cornflower), Taraxacum officinale (dandelion), Triofolium spp. -

Arthropods of Elm Fork Preserve
Arthropods of Elm Fork Preserve Arthropods are characterized by having jointed limbs and exoskeletons. They include a diverse assortment of creatures: Insects, spiders, crustaceans (crayfish, crabs, pill bugs), centipedes and millipedes among others. Column Headings Scientific Name: The phenomenal diversity of arthropods, creates numerous difficulties in the determination of species. Positive identification is often achieved only by specialists using obscure monographs to ‘key out’ a species by examining microscopic differences in anatomy. For our purposes in this survey of the fauna, classification at a lower level of resolution still yields valuable information. For instance, knowing that ant lions belong to the Family, Myrmeleontidae, allows us to quickly look them up on the Internet and be confident we are not being fooled by a common name that may also apply to some other, unrelated something. With the Family name firmly in hand, we may explore the natural history of ant lions without needing to know exactly which species we are viewing. In some instances identification is only readily available at an even higher ranking such as Class. Millipedes are in the Class Diplopoda. There are many Orders (O) of millipedes and they are not easily differentiated so this entry is best left at the rank of Class. A great deal of taxonomic reorganization has been occurring lately with advances in DNA analysis pointing out underlying connections and differences that were previously unrealized. For this reason, all other rankings aside from Family, Genus and Species have been omitted from the interior of the tables since many of these ranks are in a state of flux. -

Inter-Specific Hybridization Between Limenitis Arthemis
278 JOURNAL OF THE LEPIDOPTERISTS' SOCIETY INTER-SP ECIFIC HYBRIDIZATION BETWEEN LIMENITIS ARTHEMIS ASTYANAX AND L. ARCHIPPUS (NYMPHALIDAE) AUSTIN P. PLATT University of Maryland Baltimore County, Catonsville, Maryland and JOSEPH C. GREENFIELD, JR. Duke University Medical Center, Durham, North Carolina The Nearctic genus Limenitis (Nymphalidae) contains five common, geographically widespread forms, all of which are polytypic, and exhibit tendencies toward hybridization (Edwards, 1879; Scudder, 1889; Field, 1904; Gunder, 1934; Remington, 1958, 1968; Gage, 1970). Four of the forms are mainly allopatric in their distributions, occupying adjacent re gions, and coming in contact only along certain margins of their ranges (Hovanitz, 1949). Included among these are two conspecific eastern forms: the banded purple (L. arthemis arthemis Drury) and the red spotted purple (L. arthemis astyanax Fabricius) , an unbanded mimic of the blue swallowtail (Hattus philenor L.). In addi,tion, thcre are two western disruptively banded species: Weidemeyer's admiral (L. weide meyel'ii Edwards) and Lorquin's admiral (L. lOl'quini Boisduval). These four forms are closely allied, and conform well to Mayr's (1963) definition of a "super-species." Thc two subspecific eastern butterflies exhibit "fr.ee-interbreeding" and complete intergradation within the north castern United Statcs and southern Ontario (Edwards, 1877; Field, 1910; Hovanitz, 1949; Platt and Brower, 1968; Hcmington, 1968; Platt, Frearson, and Graves, 1970), whereas, the two western species exhibit "suturing" and "intense" interbreeding in certain restricted localities, often associated with mountain passes (Brown, 1934; Perkins and Perkins, 1966; Perkins and Perkins, 1967; Hemington, 1968). The fifth form is the predominantly orange-colored Viceroy (L. arch ippus Cramer). -
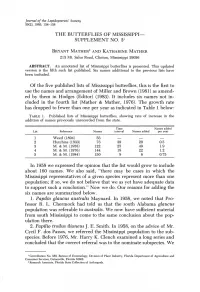
The Butterflies of Mississippi Supplement No
Journal of the Lepidopterists' Society 39(2), 1985, 134-138 THE BUTTERFLIES OF MISSISSIPPI SUPPLEMENT NO. 31 BRYANT MATHER2 AND KATHARINE MATHER 213 Mt. Salus Road, Clinton, Mississippi 39056 ABSTRACT. An annotated list of Mississippi butterflies is presented. This updated version is the fifth such list published. Six names additional to the previous lists have been included. Of the five published lists of Mississippi butterflies, this is the first to use the names and arrangement of Miller and Brown (1981) as amend ed by them in Hodges (Editor) (1983). It includes six names not in cluded in the fourth list (Mather & Mather, 1976). The growth rate has dropped to fewer than one per year as indicated in Table 1 below: TABLE 1. Published lists of Mississippi butterflies, showing rate of increase in the addition of names previously unrecorded from the state. Time Names added List Reference Names interval Names added per year 1 Weed (1894) 53 2 Hutchins (1933) 73 39 20 0.5 3 M. & M. (1958) 122 25 49 1.9 4 M. & M. (1976) 144 18 22 1.2 5 M. & M. (1984) 150 8 6 0.75 In 1958 we expressed the opinion that the list would grow to include about 160 names. We also said, "there may be cases in which the Mississippi representatives of a given species represent more than one population; if so, we do not believe that we as yet have adequate data to support such a conclusion." Now we do. Our reasons for adding the six names are summarized below. 1. -

2010 Animal Species of Concern
MONTANA NATURAL HERITAGE PROGRAM Animal Species of Concern Species List Last Updated 08/05/2010 219 Species of Concern 86 Potential Species of Concern All Records (no filtering) A program of the University of Montana and Natural Resource Information Systems, Montana State Library Introduction The Montana Natural Heritage Program (MTNHP) serves as the state's information source for animals, plants, and plant communities with a focus on species and communities that are rare, threatened, and/or have declining trends and as a result are at risk or potentially at risk of extirpation in Montana. This report on Montana Animal Species of Concern is produced jointly by the Montana Natural Heritage Program (MTNHP) and Montana Department of Fish, Wildlife, and Parks (MFWP). Montana Animal Species of Concern are native Montana animals that are considered to be "at risk" due to declining population trends, threats to their habitats, and/or restricted distribution. Also included in this report are Potential Animal Species of Concern -- animals for which current, often limited, information suggests potential vulnerability or for which additional data are needed before an accurate status assessment can be made. Over the last 200 years, 5 species with historic breeding ranges in Montana have been extirpated from the state; Woodland Caribou (Rangifer tarandus), Greater Prairie-Chicken (Tympanuchus cupido), Passenger Pigeon (Ectopistes migratorius), Pilose Crayfish (Pacifastacus gambelii), and Rocky Mountain Locust (Melanoplus spretus). Designation as a Montana Animal Species of Concern or Potential Animal Species of Concern is not a statutory or regulatory classification. Instead, these designations provide a basis for resource managers and decision-makers to make proactive decisions regarding species conservation and data collection priorities in order to avoid additional extirpations. -

LOCALIZED INTERSPECIFIC HYBRIDIZATION BETWEEN MIMETIC LIMENITIS BUTTERFLIES (NYMPHALIDAE) in FLORIDA Interspecific Hybrids Are O
Journal of the Lepidopterists' Soctety 44(3), 1990, 163-173 LOCALIZED INTERSPECIFIC HYBRIDIZATION BETWEEN MIMETIC LIMENITIS BUTTERFLIES (NYMPHALIDAE) IN FLORIDA DAVID B. RITLAND Department of Zoology, University of Florida, Gainesville, Florida 32611 ABSTRACT. Viceroy and red-spotted purple butterflies (Umenitis archippus and Limenitis arthemis ustyanax) are broadly sympatric in the eastern United States, but very rarely interbreed in most areas. However, the butterflies hybridize relatively fre quently in northern Florida and southern Georgia; I recorded seven hybrid individuals in a 13-month period in 1986-87, as well as two mating pairs of viceroy and red-spotted purple. I propose that this elevated hybridization is due to a unique combination of ecological and biogeographic (genetic) factors, which interact to locally weaken the premating reproductive barrier between viceroys and red-spotted purples. First, habitat overlap (and therefore encounter rate) between the two species of butterflies is unusually high because they share a larval foodplant. Second, red-spotted purples may be less discriminating in mate choice because of their comparative rarity (viceroy: red-spotted purple ratio is 9:1), which must affect the economics of mate choice. Finally, viceroys in northern Florida also may be prone to mismating because they represent intraspecific hybrids between two geographic races (L. a. archippus and L. a. floridensis), the latter of which is largely allopatric from red-spotted purples and may not have evolved effective pre-mating isolating mechanisms. This combination of ecological and genetic factors apparently creates a unique conduit of gene flow (introgression) between red-spotted purples and viceroys. Additional key words: Limenitis archippus, Limenitis arthemis astyanax, Salix car oliniana, introgression, mate choice. -
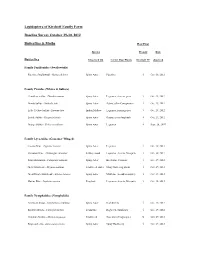
Butterflies and Moths List
Lepidoptera of Kirchoff Family Farm Baseline Survey October 29-30, 2012 Butterflies & Moths Host Plant Species Present Date Butterflies Observed On Larval Host Plants Kirchoff FF observed Family Papilionidae (Swallowtails) Pipevine Swallowtail - Battus philenor Spiny Aster Pipevine √ Oct. 30, 2012 Family Pieridae (Whites & Sulfurs) Cloudless Sulfur - Phoebis sennae Spiny Aster Legumes, clovers, peas √ Oct. 29, 2012 Dainty Sulfur - Nathalis iole Spiny Aster Asters, other Com;posites √ Oct. 29, 2012 Little Yellow Sulfur - Eurema lisa Indian Mallow Legumes, partridge pea √ Oct. 29, 2012 Lyside Sulfur - Kirgonia lyside Spiny Aster Guayacan or Soapbush √ Oct. 29, 2012 Orange Sulfur - Colias eurytheme Spiny Aster Legumes √ Sept. 26, 2007 Family Lycaenidae (Gossamer Winged) Cassius Blue - Leptotes cassius Spiny Aster Legumes √ Oct. 30, 2012 Ceraunus Blue - Hemiargus ceraunus Kidney wood Legumes- Acacia, Mesquite √ Oct. 30, 2012 Fatal Metalmark - Calephelis nemesis Spiny Aster Baccharis, Clematis √ Oct. 29, 2012 Gray Hairstreak - Strymon melinus Frostweed, Aster Many flowering plants √ Oct. 29, 2012 Great Purple Hairstreak - Atlides halesus Spiny Aster Mistletoe (in oak/mesquite) √ Oct. 29, 2012 Marine Blue - Leptotes marina Frogfruit Legumes- Acacia, Mesquite √ Oct. 30, 2012 Family Nymphalidae (Nymphalids) American Snout - Libytheana carinenta Spiny Aster Hackberries √ Oct. 29, 2012 Bordered Patch - Chlosyne lacinia Zexmenia Ragweed, Sunflower √ Oct. 29, 2012 Common Mestra - Mestra amymone Frostweed Noseburn (Tragia spp.) X Oct. 29, 2012 Empress Leila - Asterocampa leilia Spiny Aster Spiny Hackberry √ Oct. 29, 2012 Gulf Fritillary - Agraulis vanillae Spiny Aster Passion Vine X Oct. 29, 2012 Hackberry Emperor - Asterocampa celtis spiny Hackberry Hackberries √ Oct. 29, 2012 Painted Lady - Vanessa cardui Pink Smartweed Mallows & Thistles √ Oct. 29, 2012 Pearl Crescent - Phycoides tharos Spiny Aster Asters √ Oct. -

Butterflies of Tennessee Alphabetical by Common Name Butterflies Of
1 Butterflies of Tennessee Butterflies of Tennessee Alphabetical by Common Name Page 2 Butterflies of Tennessee Alphabetical by Scientific Name Page 6 Butterflies of Tennessee Alphabetical by Family Page 10 The Middle Tennessee Chapter of the North American Butterfly Association (NABA) maintains the list of Butterflies in Tennessee. Check their website at: nabamidtn.org/?page_id=176 Updated March 2015 1 2 Butterflies of Tennessee Alphabetical by Common Name Common Name Scientific Name Family American Copper Lycaena phlaeas Lycaenidae American Lady Vanessa virginiensis Nymphalidae American Snout Libytheana carinenta Nymphalidae Aphrodite Fritillary Speyeria aphrodite Nymphalidae Appalachian Azure Celestrina neglectamajor Lycaenidae Appalachian Brown Satyrodes appalachia Nymphalidae Appalachian Tiger Swallowtail Papilio appalachiensis Papilionidae Baltimore Checkerspot Euphydryas phaeton Nymphalidae Banded Hairstreak Satyrium calanus Lycaenidae Bell’s Roadside-Skipper Amblyscirtes belli Hesperiidae Black Swallowtail Papilio polyxenes Papilionidae Brazilian Skipper Calpodes ethlius Hesperiidae Broad-winged Skipper Poanes viator Hesperiidae Bronze Copper Lycaena hyllus Lycaenidae Brown Elfin Callophrys augustinus Lycaenidae Cabbage White Pieris rapae Pieridae Carolina Satyr Hermeuptychia sosybius Nymphalidae Checkered White Pontia protodice Pieridae Clouded Skipper Lerema accius Hesperiidae Clouded Sulphur Colias philodice Pieridae Cloudless Sulphur Phoebis sennae Pieridae Cobweb Skipper Hesperia metea Hesperiidae Common Buckeye Junonia coenia -

BUTTERFLIES of the Trails and Fields at Rice Creek Field Station State University of New York at Oswego
SNEAK A PEEK INSIDE... A Guide to the BUTTERFLIES of the Trails and Fields at Rice Creek Field Station State University of New York at Oswego Michael Holy - August 2010 Compton Tortoise Shell (Nymphalis vau-album) Nymphalidae Description: [L] Various shades of brown with black and white markings above. Compton Tortoise Shell Underside is a dark gray with a silver comma on its hindwing. (Nymphalis vau-album) Interesting Fact: This species is known to aestivate (“hibernate”) during the hottest Nymphalidae weeks of summer. Best Observed: Area around Herb Garden, wooded Red Trail between the upper field and parking lot. Milbert’s Tortoise Shell (Nymphalis milberti) Nymphalidae Description: [M] Orange bands across black wings above with blue spots along edge of hindwing. Milbert’s Tortoise Shell Interesting Fact: In flight this species is easily mistaken for a Comma or Question (Nymphalis milberti) Nymphalidae Mark despite its wing colors. Best Observed: Herb Garden, Beaver Meadow on Green Trail, and open fields nectaring Milkweed, Joe-pye Weed, and Purple Loosestrife, June through August. Mourning Cloak (Nymphalis antiopa) Nymphalidae Description: [L] Wings above are a rich brown black color bordered with blue spots and a pale yellow band. Mourning Cloak Interesting Fact: A true hibernator, this species can be observed in wood settings on (Nymphalis antiopa) Nymphalidae warm early spring days with snow still on the ground. Best Observed: Woods in spring, fields and all trails in summer, April through early November . White Admiral ( Limenitis arthemis) Nymphalidae Description: [L] White bands interrupt a black/brown wing color. White Admiral Interesting Fact: A variant, the Red-spotted Purple, lacks the white wing bands, (Limenitis arthemis) Nymphalidae substituting a blue green metallic hue. -

Conservation of the Arogos Skipper, Atrytone Arogos Arogos (Lepidoptera: Hesperiidae) in Florida Marc C
Conservation of the Arogos Skipper, Atrytone arogos arogos (Lepidoptera: Hesperiidae) in Florida Marc C. Minno St. Johns River Water Management District P.O. Box 1429, Palatka, FL 32177 [email protected] Maria Minno Eco-Cognizant, Inc., 600 NW 35th Terrace, Gainesville, FL 32607 [email protected] ABSTRACT The Arogos skipper is a rare and declining butterfly found in native grassland habitats in the eastern and mid- western United States. Five distinct populations of the butterfly occur in specific parts of the range. Atrytone arogos arogos once occurred from southern South Carolina through eastern Georgia and peninsular Florida as far south as Miami. This butterfly is currently thought to be extirpated from South Carolina and Georgia. The six known sites in Florida for A. arogos arogos are public lands with dry prairie or longleaf pine savanna having an abundance of the larval host grass, Sorghastrum secundum. Colonies of the butterfly are threat- ened by catastrophic events such as wild fires, land management activities or no management, and the loss of genetic integrity. The dry prairie preserves of central Florida will be especially important to the recovery of the butterfly, since these are some of the largest and last remaining grasslands in the state. It may be possible to create new colonies of the Arogos skipper by releasing wild-caught females or captive-bred individuals into currently unoccupied areas of high quality habitat. INTRODUCTION tered colonies were found in New Jersey, North Carolina, South Carolina, Florida, and Mississippi. The three re- gions where the butterfly was most abundant included The Arogos skipper (Atrytone arogos) is a very locally the New Jersey pine barrens, peninsular Florida, and distributed butterfly that occurs only in the eastern and southeastern Mississippi.