Gluconic Acid and Its Derivatives Cas N
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Oh Ho Oh Oh Oh Oh O Ho Ch3o N N H O Ho Oh Oh Oh Oh O Ho Ch3o N N
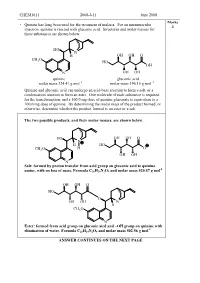
CHEM1611 2008-J-11 June 2008 Marks • Quinine has long been used for the treatment of malaria. For an intramuscular 4 injection, quinine is reacted with gluconic acid. Structures and molar masses for these substances are shown below. HO N H OH OH O CH O 3 HO OH N OH OH quinine gluconic acid molar mass 324.41 g mol –1 molar mass 196.16 g mol –1 Quinine and gluconic acid can undergo an acid-base reaction to form a salt, or a condensation reaction to form an ester. One molecule of each substance is required for the transformation, and a 160.0 mg dose of quinine gluconate is equivalent to a 100.0 mg dose of quinine. By determining the molar mass of the product formed, or otherwise, determine whether the product formed is an ester or a salt. The two possible products, and their molar masses, are shown below. HO OH OH O N H HO CH3O H O OH OH N Salt: formed by proton transfer from acid group on gluconic acid to quinine -1 amine, with no loss of mass. Formula C 26 H26 N2O9 and molar mass 520.57 g mol OH OH O HO O OH OH N H CH3O N Ester: formed from acid group on gluconic aicd and –OH group on quinine with -1 elimination of water. Formula C 26 H24 N2O8 and molar mass 502.56 g mol ANSWER CONTINUES ON THE NEXT PAGE CHEM1611 2008-J-11 June 2008 100.0 mg of quinine corresponds to: ̡̭̳̳ ̊̉̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.083 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̌̋̍.̍̊ ̧ ̭̯̬ ̊ 160.0 mg of the salt product corresponds to: ̡̭̳̳ ̊̏̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.074 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̎̋̉.̎̐ ̧ ̭̯̬ ̊ 160.0 mg of the ester product corresponds to: ̡̭̳̳ ̊̏̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.184 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̎̉̋.̎̏ ̧ ̭̯̬ ̊ As the dosages are the same, it must be the salt which is being administered. -

Some Drugs Are Excluded from Medicare Part D, but Are Covered by Your Medicaid Benefits Under the Healthpartners® MSHO Plan (HMO)
Some drugs are excluded from Medicare Part D, but are covered by your Medicaid benefits under the HealthPartners® MSHO Plan (HMO). These drugs include some over‐the‐counter (OTC) items, vitamins, and cough and cold medicines. If covered, these drugs will have no copay and will not count toward your total drug cost. For questions, please call Member Services at 952‐967‐7029 or 1‐888‐820‐4285. TTY members should call 952‐883‐6060 or 1‐800‐443‐0156. From October 1 through February 14, we take calls from 8 a.m. to 8 p.m., seven days a week. You’ll speak with a representative. From February 15 to September 30, call us 8 a.m. to 8 p.m. Monday through Friday to speak with a representative. On Saturdays, Sundays and holidays, you can leave a message and we’ll get back to you within one business day. Drug Description Strength 3 DAY VAGINAL 4% 5‐HYDROXYTRYPTOPHAN 50 MG ABSORBASE ACETAMINOPHEN 500 MG ACETAMINOPHEN 120MG ACETAMINOPHEN 325 MG ACETAMINOPHEN 650MG ACETAMINOPHEN 80 MG ACETAMINOPHEN 650 MG ACETAMINOPHEN 160 MG/5ML ACETAMINOPHEN 500 MG/5ML ACETAMINOPHEN 160 MG/5ML ACETAMINOPHEN 500MG/15ML ACETAMINOPHEN 100 MG/ML ACETAMINOPHEN 500 MG ACETAMINOPHEN 325 MG ACETAMINOPHEN 500 MG ACETAMINOPHEN 80 MG ACETAMINOPHEN 100.00% ACETAMINOPHEN 80 MG ACETAMINOPHEN 160 MG ACETAMINOPHEN 80MG/0.8ML ACETAMINOPHEN‐BUTALBITAL 50MG‐325MG ACNE CLEANSING PADS 2% ACNE TREATMENT,EXTRA STRENGTH 10% ACT ANTI‐CAVITY MOUTH RINSE 0.05% Updated 12/01/2012 ACTICAL ACTIDOSE‐AQUA 50G/240ML ACTIDOSE‐AQUA 15G/72ML ACTIDOSE‐AQUA 25G/120ML ACTIVATED CHARCOAL 25 G ADEKS 7.5 MG -

Promising Strain of Acinetobacter from Soil for Utilization of Gluconic Acid Production Topraktaki Gelecek Vaadeden Acinetobacte
S. Dinç et al. / Hacettepe J. Biol. & Chem., 2017, 45 (4), 603-607 Promising Strain of Acinetobacter from Soil for Utilization of Gluconic Acid Production Topraktaki Gelecek Vaadeden Acinetobacter Suşunun Glukonik Asit Üretiminde Kullanımı Research Article Saliha Dinç 1,2*, Meryem Kara2,3, Mehmet Öğüt3, Fatih Er1, Hacer Çiçekçi2 1Selcuk University Cumra School of Applied Sciences, Konya-Turkey. 2Selcuk Uni. Advanced Technology Research and Application Center, Konya-Turkey. 3Selcuk University Cumra Vocational High School, Konya-Turkey. ABSTRACT luconic acid, a food additive, is used in many foods to control acidity or binds metals such as calcium, Giron. Acinetobacter sp. WR326, newly isolated from soil possesses high phosphate solubilizing activity and do not require pyrroloquinoline quinone (PQQ) for glucose dehydrogenase (GDH) activity as cofactor In this study the gluconic acid production potential of this bacterium was investigated. Firstly, Acinetobacter sp. WR326 was incubated in tricalcium phosphate medium (TCP) with varying glucose concentrations (100, 250, 500 mM), at a temperature of 30°C for 5 days (120 hours). The highest gluconic acid yield (59%) was found at a glucose concentration of 100 mM. Then three different levels of gluconic acid addition to the medium (50, 100, 200 mM) were tested. When Acinetobacter sp. WR326 strain was cultivated with a 100 mM glucose and 100 mM gluconic acid the yield increased to 95.27%. In any trials 2 -keto D-gluconic acid, causes problems in processing and purification of the gluconic acid, was not detected in the medium. As a conclusion, Acinetobac- ter sp. WR326 may be considered novel potential bacterial strain for gluconic acid production. -

The Possibility of Recovering of Hydroxytyrosol from Olive Milling Wastewater by Enzymatic Bioconversion 265
ProvisionalChapter chapter 14 The PossibilityPossibility of of Recovering Recovering of Hydroxytyrosolof Hydroxytyrosol from fromOlive Milling Wastewater by Enzymatic Bioconversion Olive Milling Wastewater by Enzymatic Bioconversion Manel Hamza and Sami Sayadi Manel Hamza and Sami Sayadi Additional information is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/64774 Abstract This chapter discusses an innovative approach to obtain liquid fractions from olive mill wastewater (OMW) rich in hydroxytyrosol. The method is based on bioconversion combined with membrane separation techniques. An enzymatic bioconversion of three types of OMW was tested. TheS total volumes of OMW are 15 and 40 L. The reaction was monitored in mechanically stirred systems for 2 h at 50°C. Maximum hydroxytyr‐ osol concentrations of about 1.53, 0.83 and 0.46 g/L in the presence of 5 IU Aspergillus niger β‐glucosidase per milliliter from North OMW and South OMW were procured by two different olive millings, which are milling super press (MSP) and milling continuous chain (MCC), respectively. Enzymatic pretreatment was followed by two tangential flow membrane separation stages, microfiltration (MF) and ultrafiltration (UF). The ultrafiltration permeate was concentrated by evaporation at 45°C for 2 h. The latter exhibited a chemical oxygen demand (COD) level of 48.44 g/L. The UF permeate dehydration increased the hydroxytyrosol concentration to 7.2 g/L. A new natural product that contains some minerals beneficial to health and devoid of heavy metals or chemicals was obtained by this innovative work which describes an environmentally friendly process at pilot‐scale. -

Low Acyl Gellan Gum for Inclusion on the National List of Substances Allowed in Organic Production and Handling (7 CFR 205.605 (B)
Petition for Evaluation of Low Acyl Gellan Gum for Inclusion on the National List of Substances Allowed in Organic Production and Handling (7 CFR 205.605 (b) Submitted by: CP Kelco U.S., Inc. 3100 Cumberland Blvd., Suite 600 Atlanta, GA 30339 Date: 08 August 2019 CP Kelco U.S., Inc. 08 August 2019 National Organic List Petiion Low Acyl Gellan Gum Table of Contents Item A.1 — Section of National List ........................................................................................................... 4 Item A.2 — OFPA Category - Crop and Livestock Materials .................................................................... 4 Item A.3 — Inert Ingredients ....................................................................................................................... 4 1. Substance Name ................................................................................................................................... 5 2. Petitioner and Manufacturer Information ............................................................................................. 5 2.1. Corporate Headquarters ................................................................................................................5 2.2. Manufacturing/Processing Facility ...............................................................................................5 2.3. Contact for USDA Correspondence .............................................................................................5 3. Intended or Current Use .......................................................................................................................5 -

Sodium Gluconate and Potassium Gluconate As Substitutes for Sodium Chloride in Breadmaking
Food Sci. Technol. Res., 8 (1), 75–79, 2002 Sodium Gluconate and Potassium Gluconate as Substitutes for Sodium Chloride in Breadmaking Hiroyuki TAKANO1 and Ryouko KONDOU2 1National Food Research Institute, Tsukuba Science City, Ibaraki, 305-8642, Japan 2Fujisawa Pharmaceutical Co., LTD., 5-2-3 Tokodai, Tsukuba, Ibaraki, 300-2698, Japan Received September 5, 2001; Accepted November 30, 2001 Sodium gluconate (Na-gluconate) and potassium gluconate (K-gluconate) were used as NaCl substitutes in bread- making to determine their potential usefulness in preparing reduced-sodium bread and non-sodium bread. Replace- ment of 75% of the NaCl by Na-gluconate and of 50% by K-gluconate had no effect on rheological properties of dough as measured by Brabender Extensograph. Replacement of 100% of the NaCl by either Na-gluconate or K-glu- conate resulted in decreased resistance to extension, but the decreased resistance to extension had no effect on dough handling properties. Expansion of white bread dough (5% sugar, flour weight basis) increased with the proportion of NaCl replaced by Na-gluconate or K-gluconate. The patterns of carbon dioxide production during fermentation of non-sugar bread dough showed that as the proportion of Na-gluconate or K-gluconate increased, the time required to complete fermentation decreased, and the fermentation pattern showed a gradual resemblance to that seen in non- sugar bread dough without NaCl. In white bread, complete replacement of NaCl (2%, flour weight basis) by Na-glu- conate or K-gluconate did not cause a difference in loaf volume, nor did it have any significant effect on overall desir- ability. -

Potassium Gluconate
POTASSIUM GLUCONATE Cambridge Commodities Chemwatch Hazard Alert Code: 2 Chemwatch: 48667 Issue Date: 27/06/2017 Version No: 5.1.23.11 Print Date: 27/09/2021 Safety data sheet according to REACH Regulation (EC) No 1907/2006, as amended by UK REACH Regulations SI 2019/758 S.REACH.GB.EN SECTION 1 Identification of the substance / mixture and of the company / undertaking 1.1. Product Identifier Product name POTASSIUM GLUCONATE Chemical Name potassium gluconate CH2OH.[CH(OH)]4.CO2K; gluconic acid, potassium salt, D-; D-gluconic acid, monopotassium salt; gluconic acid, potassium salt; Synonyms potassium D-gluconate; Gluconsan K Kalium-Beta Kaon Elixir Katorin K-IAO Potalium; Potasoral Potassuril Sirokal Chemical formula C6H12O7.K Other means of P16164 identification CAS number 299-27-4 EC number 206-074-2 REACH registration 01-2119455845-28-XXXX number 1.2. Relevant identified uses of the substance or mixture and uses advised against Because potassium gluconate is almost tasteless it is a convenient for the prevent and treatment of potassium deficiency by oral Relevant identified uses administration. Uses advised against Not Applicable 1.3. Details of the supplier of the safety data sheet Registered company name Cambridge Commodities Address Lancaster Way Business Park, Ely, Cambridgeshire Cambridgeshire CB6 3NX United Kingdom Telephone +44 1353 667258 Fax Not Available Website Not Available Email [email protected] 1.4. Emergency telephone number Association / Organisation Not Available Product code: Version No: 5.1.23.2 Page 1 of 20 S.REACH.GB.EN Lancaster Way Business Park Safety Data Sheet (Conforms to Regulation (EU) No 2020/878) Ely, Cambridgeshire, CB6 3NX, UK. -

Dietary Supplements Compendium Volume 1
2015 Dietary Supplements Compendium DSC Volume 1 General Notices and Requirements USP–NF General Chapters USP–NF Dietary Supplement Monographs USP–NF Excipient Monographs FCC General Provisions FCC Monographs FCC Identity Standards FCC Appendices Reagents, Indicators, and Solutions Reference Tables DSC217M_DSCVol1_Title_2015-01_V3.indd 1 2/2/15 12:18 PM 2 Notice and Warning Concerning U.S. Patent or Trademark Rights The inclusion in the USP Dietary Supplements Compendium of a monograph on any dietary supplement in respect to which patent or trademark rights may exist shall not be deemed, and is not intended as, a grant of, or authority to exercise, any right or privilege protected by such patent or trademark. All such rights and privileges are vested in the patent or trademark owner, and no other person may exercise the same without express permission, authority, or license secured from such patent or trademark owner. Concerning Use of the USP Dietary Supplements Compendium Attention is called to the fact that USP Dietary Supplements Compendium text is fully copyrighted. Authors and others wishing to use portions of the text should request permission to do so from the Legal Department of the United States Pharmacopeial Convention. Copyright © 2015 The United States Pharmacopeial Convention ISBN: 978-1-936424-41-2 12601 Twinbrook Parkway, Rockville, MD 20852 All rights reserved. DSC Contents iii Contents USP Dietary Supplements Compendium Volume 1 Volume 2 Members . v. Preface . v Mission and Preface . 1 Dietary Supplements Admission Evaluations . 1. General Notices and Requirements . 9 USP Dietary Supplement Verification Program . .205 USP–NF General Chapters . 25 Dietary Supplements Regulatory USP–NF Dietary Supplement Monographs . -

Wöhler Synthesis of Urea
Wöhler synthesis of urea Wöhler, 1928 – – + + NH Cl + K N C O 4 NH4 N C O O H2N NH2 Friedrich Wöhler Annalen der Physik und Chemie 1828, 88, 253–256 Significance: Wöhler was the first to make an organic substance from an inorganic substance. This was the beginning of the end of the theory of vitalism: the idea that organic and inorganic materials differed essentially by the presence of the “vital force”– present only in organic material. To his mentor Berzelius:“I can make urea without thereby needing to have kidneys, or anyhow, an animal, be it human or dog" Friedrich Wöhler, 1880-1882 Polytechnic School in Berlin Support for vitalism remained until 1845, when Kolbe synthesized acetic acid Polytechnic School at Kassel from carbon disulfide University of Göttingen Fischer synthesis of glucose PhHN N O N O NHPh Br aq. HCl Δ PhNH2NH2 HO H HO H O Br "α−acrose" H OH H OH H OH H OH CH2OH CH2OH α−acrosazone α−acrosone an “osazone” (osazone test for reducing sugars) Zn/AcOH OH OH CO2H CHO O HO H HO H OH Br2 HO H HO H Na-Hg HO H HNO3 HO H H OH H OH H OH H OH then resolve H O+ H OH via strychnine H OH H OH 3 H OH CH2OH salts CH2OH CH OH CH2OH 2 D-Mannonic acid DL-Mannose DL-Mannitol DL-Fructose quinoline • Established stereochemical relationship CO2H CHO H OH H OH between mannose and glucose Na-Hg HO H HO H (part of Fischer proof) H OH + H OH H3O • work mechanisms from acrosazone on H OH H OH Emil Fischer, 1852-1919 CH2OH CH2OH University of Munich (1875-81) D-Gluconic acid D-Glucose University of Erlangen (1881-88) University of Würzburg (1888-92) University of Berlin (1892-1919) Fischer, E. -

(Punica Granatum L.) Peel Waste: a Review
molecules Review Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica granatum L.) Peel Waste: A Review Tandokazi Pamela Magangana 1,2 , Nokwanda Pearl Makunga 1 , Olaniyi Amos Fawole 3 and Umezuruike Linus Opara 2,* 1 Department of Botany and Zoology, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch 7602, South Africa; [email protected] (T.P.M.); [email protected] (N.P.M.) 2 Postharvest Technology Research Laboratory, South African Research Chair in Postharvest Technology, Department of Horticultural Sciences, Faculty of AgriSciences, Stellenbosch University, Private Bag X1, Stellenbosch 7602, South Africa 3 Postharvest Research Laboratory, Department of Botany and Plant Biotechnology, University of Johannesburg, P.O. Box 524, Auckland Park, Johannesburg 2006, South Africa; [email protected] * Correspondence: [email protected]; Tel.: +27-21-808-4064 or +27-21-808-3743 Academic Editors: Ana Barros and Irene Gouvinhas Received: 5 September 2020; Accepted: 7 October 2020; Published: 14 October 2020 Abstract: Pomegranate peel has substantial amounts of phenolic compounds, such as hydrolysable tannins (punicalin, punicalagin, ellagic acid, and gallic acid), flavonoids (anthocyanins and catechins), and nutrients, which are responsible for its biological activity. However, during processing, the level of peel compounds can be significantly altered depending on the peel processing technique used, for example, ranging from 38.6 to 50.3 mg/g for punicalagins. This review focuses on the influence of postharvest processing factors on the pharmacological, phytochemical, and nutritional properties of pomegranate (Punica granatum L.) peel. Various peel drying strategies (sun drying, microwave drying, vacuum drying, and oven drying) and different extraction protocols (solvent, super-critical fluid, ultrasound-assisted, microwave-assisted, and pressurized liquid extractions) that are used to recover phytochemical compounds of the pomegranate peel are described. -

Print This Article
Macedonian Journal of Chemistry and Chemical Engineering, Vol. 38, No. 2, pp. 149–160 (2019) MJCCA9 – 775 ISSN 1857-5552 e-ISSN 1857-5625 Received: April 23, 2019 DOI: 10.20450/mjcce.2019.1775 Accepted: October 8, 2019 Original scientific paper IDENTIFICATION AND QUANTIFICATION OF PHENOLIC COMPOUNDS IN POMEGRANATE JUICES FROM EIGHT MACEDONIAN CULTIVARS Jasmina Petreska Stanoeva1,*, Nina Peneva1, Marina Stefova1, Viktor Gjamovski2 1Institute of Chemistry, Faculty of Natural Sciences and Mathematics, Ss Cyril and Methodius University, Skopje, Republic of Macedonia 2Institute of Agriculture, Ss Cyril and Methodius University, Skopje, Republic of Macedonia [email protected] Punica granatum L. is one of the species enjoying growing interest due to its complex and unique chemical composition that encompasses the presence of anthocyanins, ellagic acid and ellagitannins, gal- lic acid and gallotannins, proanthocyanidins, flavanols and lignans. This combination is deemed respon- sible for a wide range of health-promoting biological activities. This study was focused on the analysis of flavonoids, anthocyanins and phenolic acids in eight pomegranate varieties (Punica granatum) from Macedonia, in two consecutive years. Fruits from each cultivar were washed and manually peeled, and the juice was filtered. NaF (8.5 mg) was added to 100 ml juice as a stabilizer. The samples were centrifuged for 15 min at 3000 rpm and analyzed using an HPLC/DAD/MSn method that was optimized for determination of their polyphenolic fingerprints. The dominant anthocyanin in all pomegranate varieties was cyanidin-3-glucoside followed by cya- nidin and delphinidin 3,5-diglucoside. From the results, it can be concluded that the content of anthocyanins was higher in 2016 compared to 2017. -

Catalytic Oxidation of Glucose with Hydrogen Peroxide and Colloidal Gold As Pseudo-Homogenous Catalyst
Chiang Mai J. Sci. 2016; 43(4) 825 Chiang Mai J. Sci. 2016; 43(4) : 825-833 http://epg.science.cmu.ac.th/ejournal/ Contributed Paper Catalytic Oxidation of Glucose with Hydrogen Peroxide and Colloidal Gold as Pseudo-Homogenous Catalyst: A Combined Experimental and Theoretical Investigation Jitrayut Jitonnom [a] and Christoph Sontag *[a] [a] Division of Chemistry, School of Science, University of Phayao, Phayao 56000, Thailand. *Author for correspondence; e-mail: [email protected] Received: 10 July 2013 Accepted: 25 April 2014 ABSTRACT Gold nanoparticles have been proved to act as oxidation catalyst for glucose oxidation, offering a “chemical” synthetic route to gluconic acid and gluconates - nowadays commercially produced by an enzyme catalyzed oxidation. Our investigations of the gold catalyzed oxidation route showed that gold nanoparticles produced by a modified Turkevich method have a high activity for this pseudo-homogenous catalytic reaction. Under mild reaction conditions, glucose could be oxidized in good yields (~70%) and the resulting gluconate could be isolated by column chromatography and precipitation as calcium salt. The catalytic oxidation reaction was found to follow the first-order kinetic with a rate constant of 4.95 h-1, in good agreement with previous finding. The underlying reaction mechanism is discussed, assuming that the formation of a gold-glucose cluster intermediate is a key catalytic step. Several structures of the gold-glucose intermediates were examined using density functional theory methods. The molecular behavior of glucose adsorption in gold colloid solution is present. Keywords: colloidal gold catalyst, glucose oxidation, gold nanoparticle, gluconic acid, DFT 1. INTRODUCTION Gold nanoparticles (Au NPs) as highly Catalysis by Au NPs has been emerged effective green catalysts for oxidative by the discovery of gold nanoparticles as processes have become a research topic of highly active supported catalysts for oxidation wide-spread interest in recent years [1-5], of CO into CO2 by atmospheric air [7].