Oral Care Compositions Which Comprise Stannous, Potassium and Monoflurophosphate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sodium Gluconate and Potassium Gluconate As Substitutes for Sodium Chloride in Breadmaking
Food Sci. Technol. Res., 8 (1), 75–79, 2002 Sodium Gluconate and Potassium Gluconate as Substitutes for Sodium Chloride in Breadmaking Hiroyuki TAKANO1 and Ryouko KONDOU2 1National Food Research Institute, Tsukuba Science City, Ibaraki, 305-8642, Japan 2Fujisawa Pharmaceutical Co., LTD., 5-2-3 Tokodai, Tsukuba, Ibaraki, 300-2698, Japan Received September 5, 2001; Accepted November 30, 2001 Sodium gluconate (Na-gluconate) and potassium gluconate (K-gluconate) were used as NaCl substitutes in bread- making to determine their potential usefulness in preparing reduced-sodium bread and non-sodium bread. Replace- ment of 75% of the NaCl by Na-gluconate and of 50% by K-gluconate had no effect on rheological properties of dough as measured by Brabender Extensograph. Replacement of 100% of the NaCl by either Na-gluconate or K-glu- conate resulted in decreased resistance to extension, but the decreased resistance to extension had no effect on dough handling properties. Expansion of white bread dough (5% sugar, flour weight basis) increased with the proportion of NaCl replaced by Na-gluconate or K-gluconate. The patterns of carbon dioxide production during fermentation of non-sugar bread dough showed that as the proportion of Na-gluconate or K-gluconate increased, the time required to complete fermentation decreased, and the fermentation pattern showed a gradual resemblance to that seen in non- sugar bread dough without NaCl. In white bread, complete replacement of NaCl (2%, flour weight basis) by Na-glu- conate or K-gluconate did not cause a difference in loaf volume, nor did it have any significant effect on overall desir- ability. -

Potassium Gluconate
POTASSIUM GLUCONATE Cambridge Commodities Chemwatch Hazard Alert Code: 2 Chemwatch: 48667 Issue Date: 27/06/2017 Version No: 5.1.23.11 Print Date: 27/09/2021 Safety data sheet according to REACH Regulation (EC) No 1907/2006, as amended by UK REACH Regulations SI 2019/758 S.REACH.GB.EN SECTION 1 Identification of the substance / mixture and of the company / undertaking 1.1. Product Identifier Product name POTASSIUM GLUCONATE Chemical Name potassium gluconate CH2OH.[CH(OH)]4.CO2K; gluconic acid, potassium salt, D-; D-gluconic acid, monopotassium salt; gluconic acid, potassium salt; Synonyms potassium D-gluconate; Gluconsan K Kalium-Beta Kaon Elixir Katorin K-IAO Potalium; Potasoral Potassuril Sirokal Chemical formula C6H12O7.K Other means of P16164 identification CAS number 299-27-4 EC number 206-074-2 REACH registration 01-2119455845-28-XXXX number 1.2. Relevant identified uses of the substance or mixture and uses advised against Because potassium gluconate is almost tasteless it is a convenient for the prevent and treatment of potassium deficiency by oral Relevant identified uses administration. Uses advised against Not Applicable 1.3. Details of the supplier of the safety data sheet Registered company name Cambridge Commodities Address Lancaster Way Business Park, Ely, Cambridgeshire Cambridgeshire CB6 3NX United Kingdom Telephone +44 1353 667258 Fax Not Available Website Not Available Email [email protected] 1.4. Emergency telephone number Association / Organisation Not Available Product code: Version No: 5.1.23.2 Page 1 of 20 S.REACH.GB.EN Lancaster Way Business Park Safety Data Sheet (Conforms to Regulation (EU) No 2020/878) Ely, Cambridgeshire, CB6 3NX, UK. -

Dietary Supplements Compendium Volume 1
2015 Dietary Supplements Compendium DSC Volume 1 General Notices and Requirements USP–NF General Chapters USP–NF Dietary Supplement Monographs USP–NF Excipient Monographs FCC General Provisions FCC Monographs FCC Identity Standards FCC Appendices Reagents, Indicators, and Solutions Reference Tables DSC217M_DSCVol1_Title_2015-01_V3.indd 1 2/2/15 12:18 PM 2 Notice and Warning Concerning U.S. Patent or Trademark Rights The inclusion in the USP Dietary Supplements Compendium of a monograph on any dietary supplement in respect to which patent or trademark rights may exist shall not be deemed, and is not intended as, a grant of, or authority to exercise, any right or privilege protected by such patent or trademark. All such rights and privileges are vested in the patent or trademark owner, and no other person may exercise the same without express permission, authority, or license secured from such patent or trademark owner. Concerning Use of the USP Dietary Supplements Compendium Attention is called to the fact that USP Dietary Supplements Compendium text is fully copyrighted. Authors and others wishing to use portions of the text should request permission to do so from the Legal Department of the United States Pharmacopeial Convention. Copyright © 2015 The United States Pharmacopeial Convention ISBN: 978-1-936424-41-2 12601 Twinbrook Parkway, Rockville, MD 20852 All rights reserved. DSC Contents iii Contents USP Dietary Supplements Compendium Volume 1 Volume 2 Members . v. Preface . v Mission and Preface . 1 Dietary Supplements Admission Evaluations . 1. General Notices and Requirements . 9 USP Dietary Supplement Verification Program . .205 USP–NF General Chapters . 25 Dietary Supplements Regulatory USP–NF Dietary Supplement Monographs . -
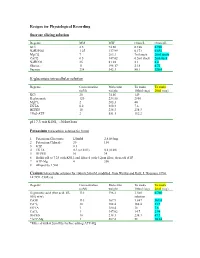
Intracellular Solution
Recipes for Physiological Recording Sucrose slicing solution Regents MM MW Gram/L Gram/4L KCl 2.5 74.56 0.186 0.746 NaH2PO4 1.25 137.99 0.173 0.692 MgCl2 7 203.3 7ml stock 28ml stock CaCl2 0.5 147.02 0.5ml stock 2ml stock NaHCO3 25 81.01 2.1 8.4 Glucose 11 198.17 2.18 8.72 Sucrose 234 342.3 80.1 320.4 K-gluconate intracellular solution Regents Concentration Molecular To make To make (mM) weight 100ml (mg) 20ml (mg) KCl 20 74.56 149 K-gluconate 120 234.56 2810 MgCl2 2 203.3 40 EGTA 0.2 380.4 7.6 HEPES 10 238.3 238.3 *Na2-ATP 2 551.1 112.2 pH 7.3 mit KOH, ~305mOsm Potassium Intracellular solution for 100ml 1. Potassium Gluconate 120mM 2,810.5mg 2. Potassium Chloride 20 150 3. GTP 0.3 4. EGTA 0.2 (or 0.02) 0.8 (0.08) 5. HEPES 10 24 6. Buffer pH to 7.25 with KOH, and filtered with 0.2μm filter, then add ATP 7. ATP-Mg 4 200 8. Aliquot by 1.5ml Cesium Intracellular solution for 100ml (243mM, modified from Weliky and Katz, J. Neurosci. 1994, 14:7291-7305) @ Regents Concentration Molecular To make To make (mM) weight 100ml (mg) 20ml (mg) D-gluconic acid (free acid, 45- 110 196.2 3.5ml 0.7ml 50% w/w) solution CsOH 110 167.9 1,847 369.4 CsCl2 10 168.4 168.4 33.7 EGTA 1 380.4 38 7.6 CaCl2 1 147.02 14.7 2.94 HEPES 10 238.3 238.3 47.7 *ATP-Mg 1 507.2 51 10.14 *Filtered with 0.2um filter before adding ATP-Mg. -

List of Annexures Annexure – I Plant Layout Annexure – II Manufacturing Process Annexure – III Details of Storage of So
List of Annexures Annexure – I Plant Layout Annexure – II Manufacturing Process Annexure – III Details of Storage of Solvents Annexure – IV Water Balance & Details of Treatment and disposal of Effluent Annexure – V Details of Treatment and Disposal of Solid Waste Annexure – VI List of Raw materials & Quantity of Consumption Annexure – VII Point Source Emissions – Stack Emission Characteristics Annexure – VIII Details of Fugitive Emissions Annexure – IX Risk Identification Annexure – X Topo Map & Administrative Set-up Map Annexures 1 GCPL ANNEXURE – I PLANT LAYOUT Annexures 2 GCPL Annexures 3 GCPL Annexures 4 GCPL ANNEXURE – II MANUFACTURING PROCESS Annexures 5 GCPL Manufacturing Process Existin g Calcium Gluconate Gluconic Acid procured from market is neutralized with Calcium carbonate, filtered, crystallised, centrifuged, dried, milled and packed. NEUTRALISATION FILTERATION CRYSTALLISATION PACKING MILLING DRYING CENTRIFUGING Alternate process for the manufacture of Calcium Gluconate Gluconic acid is neutralised with Calcium carbonate, filtered, crystallised, centrifuged, dried, milled and packed. NEUTRALISATION FILTRATION CRYSTALLISATION PACKING MILLING DRYING CENTRIFUGING Alternate process GLUCONO DELTA LACTONE + CaCO + WATER 3 FILTRATION CRYSTALLISATION (NEUTRALISATION) PACKING MILLING DRYING CENTRIFUGING Annexures 6 GCPL Sodium Gluconate Gluconic acid brought from the market is neutralised with Sodium bi-carbonate, filtered, concentrated, crystallised, centrifuged, dried, milled and packed. CONCENTRATION NEUTRALISATION FILTRATION CRYSTALLISATION -

Short-Term RCT of Increased Dietary Potassium from Potato Or
nutrients Article Short-Term RCT of Increased Dietary Potassium from Potato or Potassium Gluconate: Effect on Blood Pressure, Microcirculation, and Potassium and Sodium Retention in Pre-Hypertensive-to-Hypertensive Adults Michael S. Stone, Berdine R. Martin and Connie M. Weaver * Nutrition Science, Purdue University, West Lafayette, IN 47907, USA; [email protected] (M.S.S.); [email protected] (B.R.M.) * Correspondence: [email protected] Abstract: Increased potassium intake has been linked to improvements in cardiovascular and other health outcomes. We assessed increasing potassium intake through food or supplements as part of a controlled diet on blood pressure (BP), microcirculation (endothelial function), and potassium and sodium retention in thirty pre-hypertensive-to-hypertensive men and women. Participants were randomly assigned to a sequence of four 17 day dietary potassium treatments: a basal diet (control) of 60 mmol/d and three phases of 85 mmol/d added as potatoes, French fries, or a potassium gluconate supplement. Blood pressure was measured by manual auscultation, cutaneous microvascular and endothelial function by thermal hyperemia, utilizing laser Doppler flowmetry, and mineral retention by metabolic balance. There were no significant differences among treatments for end-of-treatment Citation: Stone, M.S.; Martin, B.R.; BP, change in BP over time, or endothelial function using a mixed-model ANOVA. However, there Weaver, C.M. Short-Term RCT of was a greater change in systolic blood pressure (SBP) over time by feeding baked/boiled potatoes Increased Dietary Potassium from compared with control (−6.0 mmHg vs. −2.6 mmHg; p = 0.011) using contrast analysis. Potassium Potato or Potassium Gluconate: Effect retention was highest with supplements. -

Submission for Potassium
MCC submission – Potassium Submission for Potassium Part A 1. International Non-proprietary Name (or British Approved Name or US Adopted Name) of the medicine. Potassium. 2. Proprietary name(s). Not applicable. 3. Name of the company / organisation / individual requesting a reclassification. Not applicable. This request is made on behalf of the natural health products industry. 4. Dose form(s) and strength(s) for which a change is sought. Not applicable. This request is made on behalf of the natural health products industry. 5. Pack size and other qualifications. Not applicable. This request is made on behalf of the natural health products industry. 6. Indications for which change is sought. Not applicable. This request is made on behalf of the natural health products industry. 7. Present classification of the medicine. At the present time, potassium is: • Unscheduled when in products for external use. • Unscheduled when in products for oral use, containing 100 mg or less. • Unscheduled in medicines for oral rehydration therapy parenteral nutrition replacement or dialysis. • Unscheduled in glucosamine sulphate complexed products containing 600 mg or less of potassium chloride per recommended dose. • Pharmacy-only medicine when in slow-release or enteric coated forms for internal use. • Pharmacy-only medicine when in medicines containing more than 100 mg per recommended dose (except in the situations described above when potassium is unscheduled) Other potassium compounds that are specifically named in the Medicines schedule that are potentially affected are: Potassium bromide Prescription medicine Page 1 of 9 MCC submission – Potassium Potassium chlorate Unscheduled when in medicines containing 10% or less, and Pharmacy-only medicine when in medicines containing more than 10% Potassium perchlorate Prescription medicine The request to increase the allowed dose of potassium does not involve the scheduling of these other specifically-named potassium substances. -

“Inert” Ingredients Used in Organic Production
“Inert” Ingredients Used in Organic Production Terry Shistar, PhD A Beyond Pesticides Report he relatively few registered pesticides allowed in organic production are contained in product formulations with so-called “inert” ingredients that are not disclosed on the T product label. The “inerts” make up the powder, liquid, granule, or spreader/sticking agents in pesticide formulations. The “inerts” are typically included in products with natural or synthetic active pesticide ingredients recommended by the National Organic Standards Board (NOSB) and listed by the National Organic Program (NOP) on the National List of Allowed and Prohibited Substances. Any of the pesticides that meet the standards of public health and environmental protection and organic compatibility in the Organic Foods Production Act (OFPA) may contain “inert” ingredients. Because the standards of OFPA are different from those used by the U.S. Environmental Protection Agency (EPA) to regulate pesticides and given changes in how the agency categorizes inerts, the NOSB has adopted a series of recommendations since 2010 that established a substance review process as part of the sunset review. NOP has not followed through on the Board’s recommendations and, as a result, there are numerous materials in use that have not been subject to OFPA criteria. This report (i) traces the history of the legal requirements for review by the NOSB, (ii) identifies the universe of toxic and nontoxic materials that make of the category of “inerts” used in products permitted in organic production, and (iii) suggests a path forward to ensure NOSB compliance with OFPA and uphold the integrity of the USDA organic label. -

Possible Prospects for Using Modern Magnesium Preparations for Increasing Stress Resistance During COVID-19 Pandemic
Research Results in Pharmacology 6(4): 65–76 UDC: 615.331 DOI 10.3897/rrpharmacology.6.59407 Review Article Possible prospects for using modern magnesium preparations for increasing stress resistance during COVID-19 pandemic Maria V. Sankova1, Olesya V. Kytko1, Renata D. Meylanova1, Yuriy L. Vasil’ev1, Michael V. Nelipa1 1 I.M. Sechenov First Moscow State Medical University (Sechenov University), N.V. Sklifosofsky Institute of Clinical Medicine, 8-2 Trubetskaya St. Moscow 119991, Russian Federation Corresponding author: Olesya V. Kytko ([email protected]) Academic editor: Tatyana Pokrovskaia ♦ Received 7 October 2020 ♦ Accepted 7 December 2020 ♦ Published 29 December 2020 Citation: Sankova MV, Kytko OV, Meylanova RD, Vasil’ev YuL, Nelipa MV (2020) Possible prospects for using modern magnesium preparations for increasing stress resistance during COVID-19 pandemic. Research Results in Pharmacology 6(4): 65–76. https://doi. org/10.3897/rrpharmacology.6.59407 Abstract Introduction: The relevance of the issue of increasing stress resistance is due to a significant deterioration in the mental health of the population caused by the special conditions of the disease control and prevention during the COVID-19 pandemic. Recently, the decisive role in the severity of clinico-physiological manifestations of maladjustment to stress is assigned to magnesium ions. The aim of the work was to study the magnesium importance in the body coping mechanisms under stress for the pathogenetic substantiation of the magnesium correction in an unfavorable situation of disease control and prevention during the COVID-19 pandemic. Materials and methods: The theoretical basis of this scientific and analytical review was an analysis of modern Russian and foreign literature data posted on the electronic portals MEDLINE, PubMed-NCBI, Scientific Electronic Library eLIBRARY.RU, Google Academy, and CyberLeninka. -

FCC 10, Second Supplement the Following Index Is for Convenience and Informational Use Only and Shall Not Be Used for Interpretive Purposes
Index to FCC 10, Second Supplement The following Index is for convenience and informational use only and shall not be used for interpretive purposes. In addition to effective articles, this Index may also include items recently omitted from the FCC in the indicated Book or Supplement. The monographs and general tests and assay listed in this Index may reference other general test and assay specifications. The articles listed in this Index are not intended to be autonomous standards and should only be interpreted in the context of the entire FCC publication. For the most current version of the FCC please see the FCC Online. Second Supplement, FCC 10 Index / Allura Red AC / I-1 Index Titles of monographs are shown in the boldface type. A 2-Acetylpyrrole, 21 Alcohol, 90%, 1625 2-Acetyl Thiazole, 18 Alcohol, Absolute, 1624 Abbreviations, 7, 3779, 3827 Acetyl Valeryl, 608 Alcohol, Aldehyde-Free, 1625 Absolute Alcohol (Reagent), 5, 3777, Acetyl Value, 1510 Alcohol C-6, 626 3825 Achilleic Acid, 25 Alcohol C-8, 933 Acacia, 602 Acid (Reagent), 5, 3777, 3825 Alcohol C-9, 922 ªAccuracyº, Defined, 1641 Acid-Hydrolyzed Milk Protein, 22 Alcohol C-10, 390 Acesulfame K, 9 Acid-Hydrolyzed Proteins, 22 Alcohol C-11, 1328 Acesulfame Potassium, 9 Acid Calcium Phosphate, 240 Alcohol C-12, 738 Acetal, 10 Acid Hydrolysates of Proteins, 22 Alcohol C-16, 614 Acetaldehyde, 11 Acidic Sodium Aluminum Phosphate, Alcohol Content of Ethyl Oxyhydrate Acetaldehyde Diethyl Acetal, 10 1148 Flavor Chemicals (Other than Acetaldehyde Test Paper, 1636 Acidified Sodium Chlorite -

Food Supplements: Permitted Vitamins and Minerals
Food Supplements List of Vitamins and Minerals which may be used in the manufacture of food supplements in the EU Digital ISBN 978 0 7504 7646 1 © Crown copyright 2012 WG15822 Important note This document was correct at the time of publishing. The lists of vitamins and minerals permitted for use in food supplements is subject to change. Please ensure you check the European Commission’s website for any amendments to the list (Click here) Vitamins and minerals which may be used in the manufacture of food supplements as permitted by Directive 2002/46/EC relating to food supplements1 Vitamins Unit of Minerals Unit of measurement measurement Vitamin A μg RE Calcium mg Vitamin D μg Magnesium mg Vitamin E mg α-TE Iron mg Vitamin K μg Copper μg Vitamin B1 mg Iodine μg Vitamin B2 mg Zinc mg Niacin mg NE Manganese mg Pantothenic acid mg Sodium mg Vitamin B6 mg Potassium mg Folic acid2 μg Selenium μg Vitamin B12 μg Chromium μg Biotin μg Molybdenum μg Vitamin C mg Fluoride mg Chloride mg Phosphorus mg Boron mg Silicon mg 1 As amended by Regulation (EC) No. 1137/2008 (OJ L311, 21.11.2008, p.1; Regulation (EC) No. 1170/2009 (OJ L314, 1.12.2009, p.36); and Regulation (EC) No. 1161/2011 (OJ L296, 15.11.2011, p.29) 2 Folic acid is the term included in Annex I of Commission Directive 2008/100/EC of 28 October 2008 amending Council Directive 90/496/EEC on nutrition labelling for foodstuffs as regards recommended daily allowances, energy conversion factors and definitions for nutrition labelling purposes and covers all forms of folates." Vitamins VITAMIN -

Folder: Product Range
Product Range Bio-based ingredients Vision From nature to ingredients ® About Jungbunzlauer Jungbunzlauer is one of the world’s leading producers of bio degra dable ingredients of natural origin. The Swiss-based, international company’s roots date back to 1867. Today, Jungbunzlauer specialises in citric acid, xanthan gum, gluconates, lactics, specialties, special salts and sweeteners for the food, beverage, pharmaceutical and cosmetic industry, as well as for various other industrial applications. Jungbunzlauer’s products are manufactured utilising natural fermentation processes based on renewable raw materials. All its products can be used, transported and disposed of in a secure and ecologically safe way. Jungbunzlauer operates manufacturing plants in Austria, Canada, France and Germany. A worldwide network of sales companies and distributors with a thorough understanding of target markets and client requirements underlies Jungbunzlauer’s strong market and customer focus. Committed to its rigorous quality standards, Jungbunzlauer guarantees for the excellence and sustainability of its products and services. With their expert knowledge, Jungbunzlauer’s Technical Service, Market Development and Application Technology teams support our customers in resolving their commercial and technical challenges with solutions tailor-made to their individual requirements and with up-to-date technical information on our products. The high-class quality of our products combines decades of experience with up-to-date know-how. Products Based on years of experience and acquired knowledge, Jungbunzlauer offers a broad spectrum of biodegradable key ingredients of natural origin to a diversified range of industries worldwide. Jungbunzlauer’s added value products are manufactured to the highest quality standards and are available in different grades with a wide variety of specifications and performances.