Folder: Product Range
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Oh Ho Oh Oh Oh Oh O Ho Ch3o N N H O Ho Oh Oh Oh Oh O Ho Ch3o N N
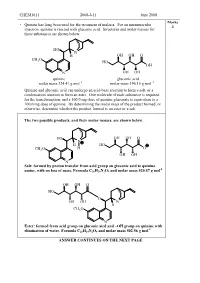
CHEM1611 2008-J-11 June 2008 Marks • Quinine has long been used for the treatment of malaria. For an intramuscular 4 injection, quinine is reacted with gluconic acid. Structures and molar masses for these substances are shown below. HO N H OH OH O CH O 3 HO OH N OH OH quinine gluconic acid molar mass 324.41 g mol –1 molar mass 196.16 g mol –1 Quinine and gluconic acid can undergo an acid-base reaction to form a salt, or a condensation reaction to form an ester. One molecule of each substance is required for the transformation, and a 160.0 mg dose of quinine gluconate is equivalent to a 100.0 mg dose of quinine. By determining the molar mass of the product formed, or otherwise, determine whether the product formed is an ester or a salt. The two possible products, and their molar masses, are shown below. HO OH OH O N H HO CH3O H O OH OH N Salt: formed by proton transfer from acid group on gluconic acid to quinine -1 amine, with no loss of mass. Formula C 26 H26 N2O9 and molar mass 520.57 g mol OH OH O HO O OH OH N H CH3O N Ester: formed from acid group on gluconic aicd and –OH group on quinine with -1 elimination of water. Formula C 26 H24 N2O8 and molar mass 502.56 g mol ANSWER CONTINUES ON THE NEXT PAGE CHEM1611 2008-J-11 June 2008 100.0 mg of quinine corresponds to: ̡̭̳̳ ̊̉̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.083 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̌̋̍.̍̊ ̧ ̭̯̬ ̊ 160.0 mg of the salt product corresponds to: ̡̭̳̳ ̊̏̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.074 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̎̋̉.̎̐ ̧ ̭̯̬ ̊ 160.0 mg of the ester product corresponds to: ̡̭̳̳ ̊̏̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.184 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̎̉̋.̎̏ ̧ ̭̯̬ ̊ As the dosages are the same, it must be the salt which is being administered. -

Second Page Vit Vs 10112011
DRUG NUTRIENT RESTORATIVE WHOLE- STANDARD PROCESS DEFICIENCY FOOD SUPPLEMENTS NATURAL ALTERNATIVES Cholesterol- Lowering Drugs Baycol, Lescol, Lipitor, Co Q10, Selenium, Zinc Cellular Vitality, Chezyn, Cyruta, Cholaplex, Livton, Mevacor, Zocor Copper Calsol, Folic Acid B12, Livaplex, Garlic 5000mg, Choline, Cataplex E, 21-Day Puriication Program, Colestid, Questran Vit A, Vit B12, Vit D, Vit E, Magnesium Lactate Tuna Omega-3 Oil, Vit K, Folic Acid, Iron, Calcium, Magnesium Lactate, Magnesium, Phosphorus, Zinc Niacinamide B6 Lopid, Tricor Coenzyme Q10, Vit E Diuretics Diuretics: Loop, Thiazide, Vit B1, Vit B6, Magnesium, Min-Tran, Calcium Lactate, A-C Carbamide, Arginex, Potassium Sparing, Misc. Potassium, Zinc, Vit C, Cataplex B, Zinc Renafood, Celery Seed 1:2, Folic Acid, Calcium Drenatrophin PMG Female Hormones Estrogen/HRT: Vit B6, Vit B12, Co Q10, Zinc Cellular Vitality, Chezyn, FemCo, Symplex F, Chaste Evista, Prempro, Folic Acid, Vit C, Magnesium, Folic Acid B12, Mag Lactate Tree, Wild Yam Complex, Premarin, Estratab Cataplex C Black Current Seed Oil, Drenamin, Hypothalmex, Neuroplex, Trace Minerals B-12 Oral Contraceptives: Vitamin B2, Vitamin B6, B6 Niacinamide, Cataplex B/G, N/A Estrastep, Norinyl, Vitamin B12, Folic Acid, Folic Acid B12, Cataplex C, Ortho-Novem, Triphasil Vitamin C, Magnesium, Zinc Magnesium Lactate, Chezyn Laxatives Potassium Organically Bound Minerals Fen-Cho, Colax, Lactic Acid Yeast, Disodium Phosphate, Magnesium Lactate Tranquilizers Major: Haldol, Vesprin Vitamin B2, Coenzyme Q10 Cellular Vitality, Cardioplus -

The Importance of Minerals in the Long Term Health of Humans Philip H
The Importance of Minerals in the Long Term Health of Humans Philip H. Merrell, PhD Technical Market Manager, Jost Chemical Co. Calcium 20 Ca 40.078 Copper 29 Cu 63.546 Iron Magnesium 26 12 Fe Mg 55.845 24.305 Manganese Zinc 25 30 Mn Zn 55.938 65.380 Table of Contents Introduction, Discussion and General Information ..................................1 Calcium ......................................................................................................3 Copper .......................................................................................................7 Iron ...........................................................................................................10 Magnesium ..............................................................................................13 Manganese ..............................................................................................16 Zinc ..........................................................................................................19 Introduction Daily intakes of several minerals are necessary for the continued basic functioning of the human body. The minerals, Calcium (Ca), Iron (Fe), Copper (Cu), Magnesium (Mg), Manganese (Mn), and Zinc (Zn) are known to be necessary for proper function and growth of the many systems in the human body and thus contribute to the overall health of the individual. There are several other trace minerals requirements. Minimum (and in some cases maximum) daily amounts for each of these minerals have been established by the Institute of -

Promising Strain of Acinetobacter from Soil for Utilization of Gluconic Acid Production Topraktaki Gelecek Vaadeden Acinetobacte
S. Dinç et al. / Hacettepe J. Biol. & Chem., 2017, 45 (4), 603-607 Promising Strain of Acinetobacter from Soil for Utilization of Gluconic Acid Production Topraktaki Gelecek Vaadeden Acinetobacter Suşunun Glukonik Asit Üretiminde Kullanımı Research Article Saliha Dinç 1,2*, Meryem Kara2,3, Mehmet Öğüt3, Fatih Er1, Hacer Çiçekçi2 1Selcuk University Cumra School of Applied Sciences, Konya-Turkey. 2Selcuk Uni. Advanced Technology Research and Application Center, Konya-Turkey. 3Selcuk University Cumra Vocational High School, Konya-Turkey. ABSTRACT luconic acid, a food additive, is used in many foods to control acidity or binds metals such as calcium, Giron. Acinetobacter sp. WR326, newly isolated from soil possesses high phosphate solubilizing activity and do not require pyrroloquinoline quinone (PQQ) for glucose dehydrogenase (GDH) activity as cofactor In this study the gluconic acid production potential of this bacterium was investigated. Firstly, Acinetobacter sp. WR326 was incubated in tricalcium phosphate medium (TCP) with varying glucose concentrations (100, 250, 500 mM), at a temperature of 30°C for 5 days (120 hours). The highest gluconic acid yield (59%) was found at a glucose concentration of 100 mM. Then three different levels of gluconic acid addition to the medium (50, 100, 200 mM) were tested. When Acinetobacter sp. WR326 strain was cultivated with a 100 mM glucose and 100 mM gluconic acid the yield increased to 95.27%. In any trials 2 -keto D-gluconic acid, causes problems in processing and purification of the gluconic acid, was not detected in the medium. As a conclusion, Acinetobac- ter sp. WR326 may be considered novel potential bacterial strain for gluconic acid production. -

Wednesday May 26, 1999
5±26±99 Vol. 64 No. 101 Wednesday Pages 28333±28712 May 26, 1999 federal register 1 VerDate 06-MAY-99 21:29 May 25, 1999 Jkt 183247 PO 00000 Frm 00001 Fmt 4710 Sfmt 4710 E:\FR\FM\26MYWS.XXX pfrm03 PsN: 26MYWS II Federal Register / Vol. 64, No. 101 / Wednesday, May 26, 1999 The FEDERAL REGISTER is published daily, Monday through SUBSCRIPTIONS AND COPIES Friday, except official holidays, by the Office of the Federal Register, National Archives and Records Administration, PUBLIC Washington, DC 20408, under the Federal Register Act (44 U.S.C. Subscriptions: Ch. 15) and the regulations of the Administrative Committee of Paper or fiche 202±512±1800 the Federal Register (1 CFR Ch. I). The Superintendent of Assistance with public subscriptions 512±1806 Documents, U.S. Government Printing Office, Washington, DC 20402 is the exclusive distributor of the official edition. General online information 202±512±1530; 1±888±293±6498 Single copies/back copies: The Federal Register provides a uniform system for making available to the public regulations and legal notices issued by Paper or fiche 512±1800 Federal agencies. These include Presidential proclamations and Assistance with public single copies 512±1803 Executive Orders, Federal agency documents having general FEDERAL AGENCIES applicability and legal effect, documents required to be published Subscriptions: by act of Congress, and other Federal agency documents of public Paper or fiche 523±5243 interest. Assistance with Federal agency subscriptions 523±5243 Documents are on file for public inspection in the Office of the Federal Register the day before they are published, unless the issuing agency requests earlier filing. -

US EPA, Inert (Other) Pesticide Ingredients in Pesticide Products
Inert Ingredients ordered by CAS Number Updated August 2004 CAS PREFIX NAME List No. 50-21-5 Lactic acid 4B 50-70-4 Sorbitol 4A 50-81-7 L- Ascorbic acid 4A 50-99-7 Dextrose 4A 51-03-6 Piperonyl butoxide 3 51-05-8 Procaine hydrochloride 3 51-55-8 Atropine 3 52-51-7 2- Bromo-2-nitro-propane-1,3-dio 3 54-21-7 Sodium salicylate 3 56-81-5 Glycerol (glycerin) 1,2,3 propanetriol 4A 56-86-0 L- Glutamic acid 3 56-95-1 Chlorhexidine diacetate 3 57-10-3 Hexadecanoic acid 4A 57-11-4 Stearic acid 4A 57-13-6 Urea 4A 57-48-7 D- Fructose 4B 57-50-1 Sugar 4A 57-55-6 Propylene glycol 4B 57-88-5 (3.beta.)- Cholest-5-en-3-ol 4B 58-08-2 1H- Purine-2,6-dione, 3,7-dihydro-1,3,7-trimethyl- 4B 58-56-0 Thiamine mononitrate 4B 58-85-5 Biotin 3 58-86-6 D- Xylose 4B 58-95-7 Vitamin E acetate 3 59-30-3 Folic acid 4B 59-40-5 N-(2- Quinoxalinyl)sulfanilide 3 59-67-6 Nicotinic acid 3 60-00-4 Ethylenediaminetetraacetic acid (EDTA) 4B 60-12-8 Benzeneethanol 3 60-29-7 Ethane, 1,1'-oxybis- 3 60-33-3 Linoleic acid 3 61-73-4 C.I. Basic Blue 9 3 62-33-9 Ethylenediaminetetraacetic acid (EDTA), calcium4B 62-54-4 Acetic acid, calcium salt 4A 63-42-3 D-(+)-Lactose 4A 63-68-3 L- Methionine 4B 64-02-8 Ethylenediaminetetraacetic acid (EDTA), tetraso4B 64-17-5 Ethanol 4B 64-18-6 Formic acid 3 64-19-7 Acetic acid 4B 64-86-8 Colchicine 3 65-85-0 Benzoic acid 4B 66-71-7 1,10- Phenanthroline 3 67-03-8 Thiamin hydrochloride 3 67-43-6 1,1,4,7,7- Diethylenetriaminepentaacetic acid 3 67-48-1 Choline chloride 4B 67-56-1 Methyl alcohol 3 67-63-0 2- Propanol 4B 67-64-1 Acetone 3 67-68-5 Dimethyl -

The Possibility of Recovering of Hydroxytyrosol from Olive Milling Wastewater by Enzymatic Bioconversion 265
ProvisionalChapter chapter 14 The PossibilityPossibility of of Recovering Recovering of Hydroxytyrosolof Hydroxytyrosol from fromOlive Milling Wastewater by Enzymatic Bioconversion Olive Milling Wastewater by Enzymatic Bioconversion Manel Hamza and Sami Sayadi Manel Hamza and Sami Sayadi Additional information is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/64774 Abstract This chapter discusses an innovative approach to obtain liquid fractions from olive mill wastewater (OMW) rich in hydroxytyrosol. The method is based on bioconversion combined with membrane separation techniques. An enzymatic bioconversion of three types of OMW was tested. TheS total volumes of OMW are 15 and 40 L. The reaction was monitored in mechanically stirred systems for 2 h at 50°C. Maximum hydroxytyr‐ osol concentrations of about 1.53, 0.83 and 0.46 g/L in the presence of 5 IU Aspergillus niger β‐glucosidase per milliliter from North OMW and South OMW were procured by two different olive millings, which are milling super press (MSP) and milling continuous chain (MCC), respectively. Enzymatic pretreatment was followed by two tangential flow membrane separation stages, microfiltration (MF) and ultrafiltration (UF). The ultrafiltration permeate was concentrated by evaporation at 45°C for 2 h. The latter exhibited a chemical oxygen demand (COD) level of 48.44 g/L. The UF permeate dehydration increased the hydroxytyrosol concentration to 7.2 g/L. A new natural product that contains some minerals beneficial to health and devoid of heavy metals or chemicals was obtained by this innovative work which describes an environmentally friendly process at pilot‐scale. -

Sodium Gluconate and Potassium Gluconate As Substitutes for Sodium Chloride in Breadmaking
Food Sci. Technol. Res., 8 (1), 75–79, 2002 Sodium Gluconate and Potassium Gluconate as Substitutes for Sodium Chloride in Breadmaking Hiroyuki TAKANO1 and Ryouko KONDOU2 1National Food Research Institute, Tsukuba Science City, Ibaraki, 305-8642, Japan 2Fujisawa Pharmaceutical Co., LTD., 5-2-3 Tokodai, Tsukuba, Ibaraki, 300-2698, Japan Received September 5, 2001; Accepted November 30, 2001 Sodium gluconate (Na-gluconate) and potassium gluconate (K-gluconate) were used as NaCl substitutes in bread- making to determine their potential usefulness in preparing reduced-sodium bread and non-sodium bread. Replace- ment of 75% of the NaCl by Na-gluconate and of 50% by K-gluconate had no effect on rheological properties of dough as measured by Brabender Extensograph. Replacement of 100% of the NaCl by either Na-gluconate or K-glu- conate resulted in decreased resistance to extension, but the decreased resistance to extension had no effect on dough handling properties. Expansion of white bread dough (5% sugar, flour weight basis) increased with the proportion of NaCl replaced by Na-gluconate or K-gluconate. The patterns of carbon dioxide production during fermentation of non-sugar bread dough showed that as the proportion of Na-gluconate or K-gluconate increased, the time required to complete fermentation decreased, and the fermentation pattern showed a gradual resemblance to that seen in non- sugar bread dough without NaCl. In white bread, complete replacement of NaCl (2%, flour weight basis) by Na-glu- conate or K-gluconate did not cause a difference in loaf volume, nor did it have any significant effect on overall desir- ability. -

Potassium Gluconate
POTASSIUM GLUCONATE Cambridge Commodities Chemwatch Hazard Alert Code: 2 Chemwatch: 48667 Issue Date: 27/06/2017 Version No: 5.1.23.11 Print Date: 27/09/2021 Safety data sheet according to REACH Regulation (EC) No 1907/2006, as amended by UK REACH Regulations SI 2019/758 S.REACH.GB.EN SECTION 1 Identification of the substance / mixture and of the company / undertaking 1.1. Product Identifier Product name POTASSIUM GLUCONATE Chemical Name potassium gluconate CH2OH.[CH(OH)]4.CO2K; gluconic acid, potassium salt, D-; D-gluconic acid, monopotassium salt; gluconic acid, potassium salt; Synonyms potassium D-gluconate; Gluconsan K Kalium-Beta Kaon Elixir Katorin K-IAO Potalium; Potasoral Potassuril Sirokal Chemical formula C6H12O7.K Other means of P16164 identification CAS number 299-27-4 EC number 206-074-2 REACH registration 01-2119455845-28-XXXX number 1.2. Relevant identified uses of the substance or mixture and uses advised against Because potassium gluconate is almost tasteless it is a convenient for the prevent and treatment of potassium deficiency by oral Relevant identified uses administration. Uses advised against Not Applicable 1.3. Details of the supplier of the safety data sheet Registered company name Cambridge Commodities Address Lancaster Way Business Park, Ely, Cambridgeshire Cambridgeshire CB6 3NX United Kingdom Telephone +44 1353 667258 Fax Not Available Website Not Available Email [email protected] 1.4. Emergency telephone number Association / Organisation Not Available Product code: Version No: 5.1.23.2 Page 1 of 20 S.REACH.GB.EN Lancaster Way Business Park Safety Data Sheet (Conforms to Regulation (EU) No 2020/878) Ely, Cambridgeshire, CB6 3NX, UK. -

Dietary Supplements Compendium Volume 1
2015 Dietary Supplements Compendium DSC Volume 1 General Notices and Requirements USP–NF General Chapters USP–NF Dietary Supplement Monographs USP–NF Excipient Monographs FCC General Provisions FCC Monographs FCC Identity Standards FCC Appendices Reagents, Indicators, and Solutions Reference Tables DSC217M_DSCVol1_Title_2015-01_V3.indd 1 2/2/15 12:18 PM 2 Notice and Warning Concerning U.S. Patent or Trademark Rights The inclusion in the USP Dietary Supplements Compendium of a monograph on any dietary supplement in respect to which patent or trademark rights may exist shall not be deemed, and is not intended as, a grant of, or authority to exercise, any right or privilege protected by such patent or trademark. All such rights and privileges are vested in the patent or trademark owner, and no other person may exercise the same without express permission, authority, or license secured from such patent or trademark owner. Concerning Use of the USP Dietary Supplements Compendium Attention is called to the fact that USP Dietary Supplements Compendium text is fully copyrighted. Authors and others wishing to use portions of the text should request permission to do so from the Legal Department of the United States Pharmacopeial Convention. Copyright © 2015 The United States Pharmacopeial Convention ISBN: 978-1-936424-41-2 12601 Twinbrook Parkway, Rockville, MD 20852 All rights reserved. DSC Contents iii Contents USP Dietary Supplements Compendium Volume 1 Volume 2 Members . v. Preface . v Mission and Preface . 1 Dietary Supplements Admission Evaluations . 1. General Notices and Requirements . 9 USP Dietary Supplement Verification Program . .205 USP–NF General Chapters . 25 Dietary Supplements Regulatory USP–NF Dietary Supplement Monographs . -

STATUTORY INSTRUMENTS. SI No. 506 of 2007
STATUTORY INSTRUMENTS. S.I. No. 506 of 2007 ———————— EUROPEAN COMMUNITIES (FOOD SUPPLEMENTS) REGULATIONS 2007 (Prn. A7/1345) 2 [506] S.I. No. 506 of 2007 EUROPEAN COMMUNITIES (FOOD SUPPLEMENTS) REGULATIONS 2007 I, MARY HARNEY, Minister for Health and Children, in exercise of the powers conferred on me by section 3 of the European Communities Act 1972 (No. 27 of 1972), and for the purpose of giving further effect to Directive 2002/46/EC1 of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food sup- plements, and for the purpose of giving effect to Commission Directive 2006/37/EC2 of 30 March 2006 amending Annex II to Directive 2002/46/EC1 of the European Parliament and of the Council as regards the inclusion of certain substances, hereby make the following regulations— PART 1 Preliminary 1. These Regulations may be cited as the European Communities (Food Supplements) Regulations 2007. 2. (1) In these Regulations— “Act of 1998” means the Food Safety Authority of Ireland Act 1998 (No. 29 of 1998); “approved examiner” means— (a) a Deputy Public Analyst, (b) an Executive Analytical Chemist, or (c) a Public Analyst, located at an official laboratory; “authorised officer” means an authorised officer appointed under section 49 of the Act of 1998; “Authority” means the Food Safety Authority of Ireland, established under section 9 of the Act of 1998; “Directive” means Directive 2002/46/EC 1 of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member 1OJ L 183, 12.7.2002, p. -

(12) Patent Application Publication (10) Pub. No.: US 2007/0287641 A1 Cassidy Et Al
US 20070287641A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0287641 A1 Cassidy et al. (43) Pub. Date: Dec. 13, 2007 (54) ACIDIC TREATMENT FLUIDS AND Publication Classification ASSOCATED METHODS (51) Int. Cl. (75) Inventors: Juanita M. Cassidy, Duncan, OK CO2F 5/10 (2006.01) (US); Jim L. Lane, Duncan, OK (US); Chad E. Kiser, Comanche, (52) U.S. Cl. ....................................................... 507/235 OK (US) Correspondence Address: (57) ABSTRACT Halliburton Energy Services, Inc. 2600 S. 2nd Street Treatment fluids that comprise a phosphorus component Duncan, OK 73536-0440 useful for inhibiting metal corrosion in acidic environments 9 and associated methods of use are provided. An example of (73) Assignee: Halliburton Energy Services, Inc. a method of using Such treatment fluids may comprise providing a treatment fluid that comprises: an aqueous base (21) Appl. No.: 11/448,945 fluid, a weak acid or salt thereof, and a phosphorus compo nent, and introducing the treatment fluid into a Subterranean (22) Filed: Jun. 7, 2006 formation. US 2007/0287641 A1 Dec. 13, 2007 ACDIC TREATMENT FLUIDS AND lems, resort to using corrosion inhibitor formulations that ASSOCATED METHODS may be less effective, or forego the use of certain acidic treatment fluids entirely. BACKGROUND SUMMARY 0001. The present invention relates to methods and com 0006. The present invention relates to methods and com positions for treating Subterranean formations. More par positions for treating Subterranean formations. More par ticularly, the present invention relates to treatment fluids that ticularly, the present invention relates to treatment fluids that comprise a phosphorus component useful, inter alia, for comprise a phosphorus component useful, inter alia, for inhibiting metal corrosion in acidic environments, and asso inhibiting metal corrosion in acidic environments, and asso ciated methods of use.