Antioxidative and Anti-Histamine-Release Activities of Excoecaria Agallocha L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stillingia: a Newly Recorded Genus of Euphorbiaceae from China
Phytotaxa 296 (2): 187–194 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2017 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.296.2.8 Stillingia: A newly recorded genus of Euphorbiaceae from China SHENGCHUN LI1, 2, BINGHUI CHEN1, XIANGXU HUANG1, XIAOYU CHANG1, TIEYAO TU*1 & DIANXIANG ZHANG1 1 Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China 2University of Chinese Academy of Sciences, Beijing 100049, China * Corresponding author, email: [email protected] Abstract Stillingia (Euphorbiaceae) contains ca. 30 species from Latin America, the southern United States, and various islands in the tropical Pacific and in the Indian Ocean. We report here for the first time the occurrence of a member of the genus in China, Stillingia lineata subsp. pacifica. The distribution of the genus in China is apparently narrow, known only from Pingzhou and Wanzhou Islands of the Wanshan Archipelago in the South China Sea, which is close to the Pearl River estuary. This study updates our knowledge on the geographic distribution of the genus, and provides new palynological data as well. Key words: Island, Hippomaneae, South China Sea, Stillingia lineata Introduction During the last decade, hundreds of new plant species or new species records have been added to the flora of China. Nevertheless, newly described or newly recorded plant genera are not discovered and reported very often, suggesting that botanical expedition and plant survey at the generic level may be advanced in China. As far as we know, only six and eight angiosperm genera respectively have been newly described or newly recorded from China within the last ten years (Qiang et al. -

Excoecaria Agallocha L. Antimicrobial Properties Against Important Pathogenic Microorganisms
International Journal of ChemTech Research CODEN( USA): IJCRGG ISSN : 0974-4290 Vol.1, No.4, pp 865-867, Oct-Dec 2009 EXCOECARIA AGALLOCHA L. ANTIMICROBIAL PROPERTIES AGAINST IMPORTANT PATHOGENIC MICROORGANISMS Varahalarao Vadlapudi1, Varaprasad Bobbarala1*, Somasekhar Penumajji2, K. Chandrasekhar Naidu1 1Department of Botany, Andhra University, Visakhapatnam-3, A.P.,India. 2Vivimed labs Limited, 2nd, 4th Floor, Veeranag towers, Habsiguda, Hyderabad, A.P.,India. * Corresponding Author: [email protected] ABSTRACT: Excoecaria agallocha L. leaves were extracted by various extracting procedures, using different solvents for testing the antimicrobial activities against important microorganisms using agar well diffusion method. Chloroform and methanolic extracts were found to be effective against these organisms, whereas hexane extracts were inactive. The purpose of this study was to find preliminary data for the development of alternative treatments to chemical microbicides for the control of plant diseases from natural plant extracts. Keywords: Excoecaria agallocha, Agar well diffusion method; Antimicrobial activity. INTRODUCTION phytochemical and bioactivity studies on mangrove Medical plants have been used for years in daily plants from Kakinada and Godavari, we now report life to treat disease all over the world. It is well known assessment of in vitro antimicrobial activity including that some plants containing active compounds are able to pathogenic bacterial and fungal strains. inhibit the microbial growth. The potential of antimicrobial properties of plants are related to their MATERIALS AND METHODS ability to synthesize compounds by the secondary E. agallocha L. commonly known as milky metabolism. Secondary metabolites proved to be the most mangrove and its vernacular name is Tilla and this important group of compounds that showed wide range species of mangrove tree classified in the plant family of antibacterial and antifungal activity. -
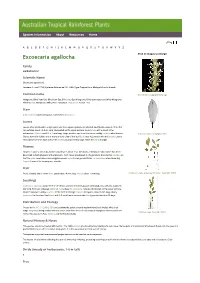
Excoecaria Agallocha Click on Images to Enlarge
Species information Abo ut Reso urces Hom e A B C D E F G H I J K L M N O P Q R S T U V W X Y Z Excoecaria agallocha Click on images to enlarge Family Euphorbiaceae Scientific Name Excoecaria agallocha L. Linnaeus, C. von (1759) Systema Naturae ed. 10 : 1288. Type: Tropical Asia, Malaya & Pacific Islands. Common name Male flowers. Copyright Barry Jago Mangrove, Blind Your Eye; Blind Your Eye; Blind Your Eye Mangrove; Blind-your-eyes-tree; Milky Mangrove; Blinding Tree; Mangrove, Milky; River Poisonous Tree; Scrub Poison Tree Stem Bark exudate rapid and copious. Sometimes deciduous. Leaves Leaves often produced in a tight spiral and thus appear opposite or whorled. Leaf blades about 6-10.5 x 3-5 cm, petioles about 1.5-2 cm long, channelled on the upper surface. Apex retuse with a gland in the indentation. Stipules small, 1.5-2 mm long. Twigs, petioles and leaves produce a milky exudate when broken. Scale bar 10mm. Copyright CSIRO Glands normally visible, one or more on each side of the leaf blade near its junction with the petiole. Lateral veins about 12-18 on each side of the midrib usually forming loops inside the blade margin. Flowers Flowers in spikes, about 40-70 mm long, flowers about 1.5-2 mm diam., emitting an odour which has been described as both pleasant and unpleasant. Each flower enveloped in a bi-glandular bract before anthesis so that the spike resembles a narrow gymnosperm cone. Pollen yellow. Plants +/- deciduous when flowering. -

Fl. China 11: 280–282. 2008. 65. EXCOECARIA Linnaeus, Syst. Nat
Fl. China 11: 280–282. 2008. 65. EXCOECARIA Linnaeus, Syst. Nat., ed. 10, 2: 1288. 1759. 海漆属 hai qi shu Li Bingtao (李秉滔 Li Ping-tao); Hans-Joachim Esser Commia Loureiro. Trees or shrubs, with milky juice, glabrous. Leaves alternate or opposite, petiolate; stipules small, caducous; leaf blade entire or serrulate, penninerved. Flowers unisexual (plants monoecious or dioecious), apetalous, without disk, in axillary or terminal racemelike thyrses. Male flowers (sub)sessile; sepals 3, small, imbricate, free; stamens 3; filaments free; anthers longitudinally dehiscent, without pistillode. Female flowers sessile to pedicellate; calyx 3-lobed or 3-partite; ovary 3-celled, smooth; ovules 1 per locule; stigmas extended or recurved, free to slightly connate at base, undivided, eglandular. Capsules dehiscent into 2-valved cocci; columella persistent, winged. Seeds globose, estrophiolate; episperm crustaceous; endosperm fleshy; cotyledon broad and flattened. About 35 species: Africa, Asia, Australia, Pacific islands; five species (two endemic) in China. 1a. Leaves opposite above, alternate on lower parts. 2a. Leaf blade purple or dark red abaxially........................................................................................................ 1. E. cochinchinensis 2b. Leaf blade green or greenish abaxially when old. 3a. Leaf blade ca. 3 × as long as wide, apex acuminate, not falcate, petiole 3–13 mm ............................ 1. E. cochinchinensis 3b. Leaf blade more than 5 × as long as wide, apex acuminate-falcate, petiole 3–5 mm .................................... 2. E. venenata 1b. Leaves alternate on all parts. 4a. Leaf blade serrulate; male bracts 2- or 3-flowered ............................................................................................... 5. E. acerifolia 4b. Leaf blade entire or nearly so; male bracts 1-flowered. 5a. Petioles 2-glandular at apex; plants dioecious ................................................................................................ 3. E. agallocha 5b. -

Fl. China 11: 284–285. 2008. 69. TRIADICA Loureiro, Fl. Cochinch. 2
Fl. China 11: 284–285. 2008. 69. TRIADICA Loureiro, Fl. Cochinch. 2: 598, 610. 1790. 乌桕属 wu jiu shu Li Bingtao (李秉滔 Li Ping-tao); Hans-Joachim Esser Sapium sect. Triadica (Loureiro) Müller Argoviensis. Trees or shrubs, monoecious or sometimes dioecious; indumentum absent; latex white. Leaves alternate or nearly opposite; petioles with 1 or 2 apical glands; leaf blade margin entire or serrate; venation pinnate, lowermost pair of veins originating at very leaf base, forming basal margin. Inflorescences terminal or axillary, spikelike or racemelike thyrses, sometimes branched; bracts with 2 large abaxial glands at base. Male flowers small, yellow, fascicled in axils of bracts; calyx membranous, cup-shaped, shallowly 2- or 3-lobed or -dentate; petals absent; disk absent; stamens 2–3; filaments free; anthers 2-celled, longitudinally dehiscent; pistillode absent. Female flowers larger than male, 1 per bract; calyx cup-shaped, 3-partite, or cylindric and 3-dentate, rarely 2- or 3-sepaled; petals absent; disk absent; ovary 2- or 3-celled; ovules 1 per cell; styles usually 3, free or connate at base; stigma revolute, entire. Capsules globose, pyriform or 3-valved, rarely baccate, usually 3-celled, loculicidal, sometimes irregularly dehiscent. Seeds subglobose, usually covered by waxy aril; exocarp hard; endosperm fleshy; cotyledon broad and flattened. Three species: E and S Asia; three species in China. 1a. Petiole with a single gland above; leaf blade subrotund, base cordate to rounded, apex rounded or rarely acute or incised .................................................................................................................................................................... 3. T. rotundifolia 1b. Petiole with a pair of glands above; leaf blade ovate to elliptic, base cuneate to obtuse (very rarely rounded), apex acute to acuminate. -

Downloaded from Brill.Com10/09/2021 12:24:23AM Via Free Access 2 IAWA Journal, Vol
IAWA Journal, Vol. 26 (1), 2005: 1-68 WOOD ANATOMY OF THE SUBFAMILY EUPHORBIOIDEAE A comparison with subfamilies Crotonoideae and Acalyphoideae and the implications for the circumscription of the Euphorbiaceae Alberta M. W. Mennega Nationaal Herbarium Nederland, Utrecht University branch, Heidelberglaan 2, 3584 es Utrecht, The Netherlands SUMMARY The wood anatomy was studied of 82 species from 34 out of 54 genera in the subfamily Euphorbioideae, covering all five tribes recognized in this subfamily. In general the woods show a great deal of similarity. They are charac terized by a relative paucity of vessels, often arranged in short to long, dumbbell-shaped or twin, radial multiples, and by medium-sized to large intervessel pits; fibres often have gelatinous walls; parenchyma apotracheal in short, wavy, narrow bands and diffuse-in-aggregates; mostly uni- or only locally biseriate rays, strongly heterocellular (except Hippomane, Hura and Pachystroma). Cell contents, either silica or crystals, or both together, are nearly always present and often useful in distinguishing between genera. Radiallaticifers were noticed in most genera, though they are scarce and difficult to trace. The laticifers are generally not surrounded by special cells, except in some genera of the subtribe Euphorbiinae where radiallaticifers are comparatively frequent and conspicuous. Three ofthe five tribes show a great deal of conformity in their anatomy. Stomatocalyceae, however, stand apart from the rest by the combination of the scarcity of vessels, and mostly biseriate, vertically fused and very tall rays. Within Euphorbieae the subtribe Euphorbiinae shows a greater vari ation than average, notably in vessel pitting, the frequent presence of two celled parenchyma strands, and in size and frequency of the laticifers. -

Insight on Excoecaria Agallocha
s Chemis ct try u d & o r R P e s l e Kaliamurthi and Selvaraj, Nat Prod Chem Res 2016, 4:2 a r a r u t c h a N Natural Products Chemistry & Research DOI: 10.4172/2329-6836.1000203 ISSN: 2329-6836 Research Article Open Access Insight on Excoecaria agallocha: An Overview Satyavani Kaliamurthi* and Gurudeeban Selvaraj Centre of Advanced Study in Marine Biology, Annamalai University, Tamil Nadu, India Abstract Excoecaria agallocha is a milky mangrove widely distributed in Indian coastal regions. This review article explains chemical composition, pharmaceutical and environmental applications of E. agallocha. There are 20 different polyphenols, 15 terpenoids and more than 50 volatile derivatives were identified from leaves, stem, latex and root extract. Enormous number of compounds isolated from ethanolic extact of leaves. In conclusion, E. agallocha has huge amount of polyphenols and terpenoids, which was reported to have endocrine, epidemic and endemic disease control as anti-microbial, anti-cancer and anti-diabetic agent. Keywords: Mangroves; Thillai; Terpenoids; Rutin; Antidiabetic insulin and glucagon. The islets of Langerhans secrete insulin and glucagon directly into the blood [7]. When the blood glucose level falls, Background glucagon secreted and increases blood glucose concentration partly by A mangrove is a tree, shrub, palm or ground fern, generally breaking down stored glycogen in the liver by a glycogenolysis pathway. exceeding one half meter in height, that normally grows above mean Also, Gluconeogenesis is the production of glucose in the liver from sea level in the intertidal zone of marine coastal environments and non-carbohydrate precursors such as glycogenic amino acids [8]. -

The Tribe Hippomaneae (Euphorbiaceae) in Brazil a Tribo Hippomaneae (Euphorbiaceae) No Brasil
Rodriguésia 63(1): 209-225. 2012 http://rodriguesia.jbrj.gov.br The tribe Hippomaneae (Euphorbiaceae) in Brazil A tribo Hippomaneae (Euphorbiaceae) no Brasil Hans-Joachim Esser1 Abstract The tribe Hippomaneae (Euphorbiaceae) in Brazil. The tribe Hippomaneae is discussed with respect to its taxonomic history, its placement within the Euphorbiaceae, its diagnostic characters (particularly the floral buds), current data on phylogeny and subdivision, and its general pattern of diversity. The tribe is represented in Brazil with 13 genera and ca. 120 species. A key to the Brazilian genera is provided. All Brazilian genera are discussed, citing relevant characters, recent taxonomic literature, and the current state of knowledge, sometimes pointing to unresolved problems. For five of the genera, published revisions exist; six genera have unpublished but completed revisions or are currently under revision. Actinostemon and Gymnanthes are currently the most difficult genera, mostly based on the absence of available up-to-date taxonomic references. For Mabea and Senefeldera, two genera with completed but currently unpublished revisions, additional data are given on aspects of their taxonomy, ecology and biogeography. Key words: Brazilian Hippomaneae, Mabea, Senefeldera, flowering plant taxonomy. Resumo A tribo Hippomaneae é discutida em relação à sua história taxonômica, posição sistemática nas Euphorbiaceae, em seus principais caracteres morfológicos diagnósticos, com ênfase no botão floral, em sua atual filogenia e subdivisão, e em seus padrões gerais de diversidade. A tribo está representada no Brasil por 13 gêneros e cerca de 120 espécies. Uma chave para os gêneros brasileiros é fornecida. Todos os gêneros do Brasil são discutidos sucintamente, citando-se suas características mais relevantes, a literatura taxonômica mais recente e o seu estado atual de conhecimento, bem como algumas sugestões para problemas ainda não resolvidos sobre os táxons. -

Journal Arnold Arboretum
JOURNAL OF THE ARNOLD ARBORETUM HARVARD UNIVERSITY G. SCHUBERT T. G. HARTLEY PUBLISHED BY THE ARNOLD ARBORETUM OF HARVARD UNIVERSITY CAMBRIDGE, MASSACHUSETTS DATES OF ISSUE No. 1 (pp. 1-104) issued January 13, 1967. No. 2 (pp. 105-202) issued April 16, 1967. No. 3 (pp. 203-361) issued July 18, 1967. No. 4 (pp. 363-588) issued October 14, 1967. TABLE OF CONTENTS COMPARATIVE MORPHOLOGICAL STUDIES IN DILLENL ANATOMY. William C. Dickison A SYNOPSIS OF AFRICAN SPECIES OF DELPHINIUM J Philip A. Munz FLORAL BIOLOGY AND SYSTEMATICA OF EUCNIDE Henry J. Thompson and Wallace R. Ernst .... THE GENUS DUABANGA. Don M. A. Jayaweera .... STUDIES IX SWIFTENIA I MKUACKAE) : OBSERVATION UALITY OF THE FLOWERS. Hsueh-yung Lee .. SOME PROBLEMS OF TROPICAL PLANT ECOLOGY, I Pompa RHIZOME. Martin H. Zimmermann and P. B Two NEW AMERICAN- PALMS. Harold E. Moure, Jr NOMENCLATURE NOTES ON GOSSYPIUM IMALVACE* Brizicky A SYNOPSIS OF THE ASIAN SPECIES OF CONSOLIDA CEAE). Philip A. Munz RESIN PRODUCER. Jean H. Langenheim COMPARATIVE MORPHOLOGICAL STUDIES IN DILLKNI POLLEN. William C. Dickison THE CHROMOSOMES OF AUSTROBAILLVA. Lily Eudi THE SOLOMON ISLANDS. George W. G'dUtt A SYNOPSIS OF THE ASIAN SPECIES OF DELPII STRICTO. Philip A. Munz STATES. Grady L. Webster THE GENERA OF EUPIIORBIACEAE IN THE SOT TUFA OF 1806, AN OVERLOOI EST. C. V. Morton REVISION OF THE GENI Hartley JOURNAL OF THE ARNOLD ARBORETUM HARVARD UNIVERSITY T. G. HARTLEY C. E. WOOD, JR. LAZELLA SCHWARTEN Q9 ^ JANUARY, 1967 THE JOURNAL OF THE ARNOLD ARBORETUM Published quarterly by the Arnold Arboretum of Harvard University. Subscription price $10.00 per year. -

(Euphorbiaceae) (1807) As Stillingia Populnea. Only By
BLUMEA 42 (1997) 421-466 A revision of Omalanthus (Euphorbiaceae) in Malesia H.-J. Esser Rijksherbarium/Hortus Botanicus, P.O. Box 9514, 2300 RA Leiden, The Netherlands Summary Omalanthus is revised for Malesia and the Solomon Islands. For this region, 13 species without subspecific taxa are accepted, namely O. arfakiensis, O. caloneurus, O. fastuosus, O. giganteus, O. grandifolius, O. longistylus, O. macradenius, O. nervosus, O. novoguineensis, O. populifolius, O. O. O. In addition populneus, and trivalvis, and, as a new species, remotus. to established syno- not nyms, 17 species from Malesia are accepted any more. The diagnostical importance of a peltate or non-peltate leaf base is especially doubted. Although detailed phylogenetic and biogeographical analyses were not made, the value of the established sections of Omalanthus is questioned. An alter- native subdivision unites all Malesian taxa into four subgroups, each with a distinct distribution of probably historic causes.The subgroups are distributed in W and C Malesia, the Lesser Sunda Is- lands, the Philippines and the Bismarck Archipelago, and New Guinea and Australia, respectively. The radiation in and habitats also have contributed the actual montane subalpine may to diversity. In O. from French is be of O. stokesii. addition, gracilis Polynesia proposed to a synonym Introduction Omalanthus was first mentioned as Duania by Noronha (1790), then as Carumbium by Reinwardt (1823) and finally as Omalanthus by De Jussieu (1824). Neither Du- ania nor Carumbium were accompanied by diagnostical information and, therefore, Carumbium later Reinwardt not validly published. was validated by (1825) through a detailed description, but this had been preceeded by De Jussieu (1824). -

443566346002.Pdf
Revista Cubana de Química ISSN: 2224-5421 Universidad de Oriente Arró-Díaz, Diana Julia; Castelnaux-Ochoa, Naylan; Ochoa-Pacheco, Ania; Do-Nascimento, Yuri Mangueira Antioxidant activity of bioactive compounds isolated from leaves and bark of Gymnanthes lucida Sw Revista Cubana de Química, vol. 33, no. 1, 2021, pp. 22-39 Universidad de Oriente Available in: http://www.redalyc.org/articulo.oa?id=443566346002 How to cite Complete issue Scientific Information System Redalyc More information about this article Network of Scientific Journals from Latin America and the Caribbean, Spain and Journal's webpage in redalyc.org Portugal Project academic non-profit, developed under the open access initiative Rev. Cubana Quím. Vol.33, no.1 enero-abril, 2021, e-ISSN: 2224-5421 Artículo original Actividad antioxidante de compuestos bioactivos aislados de la hoja y corteza de Gymnanthes lucida Sw Antioxidant activity of bioactive compounds isolated from leaves and bark of Gymnanthes lucida Sw Diana Julia Arró-Díaz1* http://orcid.org/0000-0002-3379-0956. Naylan Castelnaux-Ochoa2 http://orcid.org/0000-0002-3826-9043. Ania Ochoa-Pacheco3 http://orcid.org/0000-0002-1028-6626. Yuri Mangueira-Do Nascimento4 http://orcid.org/0000-0002-9732-6191. 1Center of Toxicology and Biomedicine (TOXIMED), Santiago de Cuba, Cuba 2Biological-Pharmaceutical Laboratories (LABIOFAM) Enterprise, Santiago de Cuba, Cuba 3Pharmacy Department, Faculty of Natural Sciences, Oriente University, Santiago de Cuba, Cuba 4Posgraduate Program of Bioactive Sintetic and Natural Products. Health Sciences Institute. Federal University of Paraíba, Brazil *Autor para correspondencia: correo electrónico: [email protected] 22 RESUMEN Estudios fitoquímicos sugieren que Gymnanthes lucida Sw. (aité) es un candidato con potencial antioxidante. -

A Botanical Gordian Knot: the Case of Ateramnus and Gymnanthes (Euphorbiaceae)
A BOTANICAL GORDIAN KNOT: THE CASE OF ATERAMNUS AND GYMNANTHES (EUPHORBIACEAE) Grady L. Webster' The taxa of the Euphorbiaceous tribe Hippomaneae (sensu Webster, 1975) still present notoriously difficult problems in generic delimitation, despite the revisionary efforts of Mueller (1866), Baillon (1874), Bentham (1880), Pax and Hoffmann (1912), and others. This is especially true of the complex of taxa with highly reduced flowers which Baillon (1874) reduced to the single genus Excoecaria. Although Rogers (1951) achieved an acceptable differentiation between Sapium and Stillingia, the demarcation lines between Actinostemon, Gymnanthes, Sebastiania, and some smaller genera remain uncertain. This problem in classification is exacerbated by some nomenclatural difficulties, among which the status of Gymnanthes is most critical. Rothmaler (1944) initiated a still unresolved nomenclatural crisis by resurrecting the generic name Ateramnus P. Browne (1756), based on a Jamaican plant that Rothmaler identified with Gymnanthes lucida Sw. Since Ateramnus is a validly published earlier name, Rothmaler argued that it should be adapted for the genus currently known as Gymnanthes Sw. (1788). Recently, this suggestion of Roth- maler's has been taken up by Adams (1970, 1972) and by Gillis (1974), who apply names under Ateramnus to species heretofore treated under Gymnanthes. Gillis avowedly follows the lead of Dandy (1967), who in his index of generic names published between 1753 and 1774 listed Ateramnus in such a manner as to imply that it has priority over Gymnanthes. This has created a confusing situation in the current literature, because some botanists have followed Adams and Gillis in adopting Ateramnus (Correll, 1979; Tomlinson, 1980), whereas others continue to use Gymnanthes (Foumet, 1978; Elias, 1980).