Switching from Trimipramine to Sertraline
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cambridgeshire and Peterborough Joint Prescribing Group MEDICINE REVIEW
Cambridgeshire and Peterborough Joint Prescribing Group MEDICINE REVIEW Name of Medicine / Trimipramine (Surmontil®) Class (generic and brand) Licensed indication(s) Treatment of depressive illness, especially where sleep disturbance, anxiety or agitation are presenting symptoms. Sleep disturbance is controlled within 24 hours and true antidepressant action follows within 7 to 10 days. Licensed dose(s) Adults: For depression 50-75 mg/day initially increasing to 150-300 mg/day in divided doses or one dose at night. The maintenance dose is 75-150 mg/day. Elderly: 10-25 mg three times a day initially. The initial dose should be increased with caution under close supervision. Half the normal maintenance dose may be sufficient to produce a satisfactory clinical response. Children: Not recommended. Purpose of Document To review information currently available on this class of medicines, give guidance on potential use and assign a prescribing classification http://www.cambsphn.nhs.uk/CJPG/CurrentDrugClassificationTable.aspx Annual cost (FP10) 10mg three times daily: £6,991 25mg three times daily: £7,819 150mg daily: £7,410 300mg daily: £14,820 Alternative Treatment Options within Class Tricyclic Annual Cost CPCCG Formulary Classification Antidepressant (FP10) Amitriptyline (75mg) Formulary £36 Lofepramine (140mg) Formulary £146 Imipramine (75mg) Non-formulary £37 Clomipramine (75mg) Non-formulary £63 Trimipramine (75mg). TBC £7,819 Nortriptyline (75mg) Not Recommended (pain) £276 Doxepin (150mg) TBC £6,006 Dosulepin (75mg) Not Recommended (NICE DO NOT DO) £19 Dosages are based on possible maintenance dose and are not equivalent between medications Recommendation It is recommended to Cambridgeshire and Peterborough CCG JPG members and through them to local NHS organisations that the arrangements for use of trimipramine are in line with restrictions agreed locally for drugs designated as NOT RECOMMENDED:. -

Psychotropic Medications Judicial Reference Guide
PSYCHOTROPIC MEDICATIONS JUDICIAL REFERENCE GUIDE (Revised Edition 7/15/10) PSYCHOTROPIC MEDICATIONS JUDICIAL REFERENCE GUIDE FIRST EDITION THE STEERING COMMITTEE ON FAMILIES AND CHILDREN IN THE COURT Distributed by Florida Supreme Court 500 South Duval Street Tallahassee, FL 32399-1900 (850) 488-0125 INTRODUCTION One of the toughest challenges facing our dependency courts is the mental health of our children. “In July 2003, the Florida Statewide Advocacy Council published a Red Item Report finding 55% of foster children…in the state of Florida had been put on powerful mind altering psychotropic drugs.”1 In order to assist in this regard, the Psychotherapeutic Medication Subcommittee of the Steering Committee on Families and Children in the Court of the Supreme Court of Florida compiled this resource guide to help judges have a better understanding of psychotropic medications and their interaction with other drugs and with mental health disorders. Recently, the tragic case of Gabriel Myers in 2009 highlighted the fact that a number of child deaths were linked to the off label use of anti-psychotic medications. This is of special concern to Dependency Judges who are ultimately responsible for children in Florida’s Foster Care system. The researchers used publically available data from the internet, FDA manufactures’ published guidelines, publically available non-copyrighted articles and Dr. Brenda Thompson graciously prepared the Psychotropic Medication Chart. Special thanks to Dr. Brenda Thompson, the Honorable Herbert J. Baumann, the Honorable Ralph C. Stoddard, General Magistrate Tracy Ellis, Avron Bernstein, Selena Schoonover, Daniel Ringhoff, Jovasha Lang and to the Members of the Psychotherapeutic Medication Subcommittee. -

Decomposition Profile Data Analysis of Multiple Drug Effects Identifies
www.nature.com/scientificreports OPEN Decomposition profle data analysis of multiple drug efects identifes endoplasmic reticulum stress‑inducing ability as an unrecognized factor Katsuhisa Morita1,2, Tadahaya Mizuno1,2* & Hiroyuki Kusuhara1* Chemicals have multiple efects in biological systems. Because their on‑target efects dominate the output, their of‑target efects are often overlooked and can sometimes cause dangerous adverse events. Recently, we developed a novel decomposition profle data analysis method, orthogonal linear separation analysis (OLSA), to analyse multiple efects. In this study, we tested whether OLSA identifed the ability of drugs to induce endoplasmic reticulum (ER) stress as a previously unrecognized factor. After analysing the transcriptome profles of MCF7 cells treated with diferent chemicals, we focused on a vector characterized by well‑known ER stress inducers, such as ciclosporin A. We selected fve drugs predicted to be unrecognized ER stress inducers, based on their inducing ability scores derived from OLSA. These drugs actually induced X‑box binding protein 1 splicing, an indicator of ER stress, in MCF7 cells in a concentration‑dependent manner. Two structurally diferent representatives of the fve test compounds exhibited similar results in HepG2 and HuH7 cells, but not in PXB primary hepatocytes derived from human‑liver chimeric mice. These results indicate that our decomposition strategy using OLSA uncovered the ER stress‑inducing ability of drugs as an unrecognized efect, the manifestation of which depended on the background of the cells. Small compounds have the capacity to interact with multiple cellular proteins, thereby mediating multiple efects depending on their exposure to interacting proteins. It is quite difcult to identify the full range of efects of a new chemical, even for its developers, and some factors may be unrecognized. -

CENTRAL NERVOUS SYSTEM DEPRESSANTS Opioid Pain Relievers Anxiolytics (Also Belong to Psychiatric Medication Category) • Codeine (In 222® Tablets, Tylenol® No
CENTRAL NERVOUS SYSTEM DEPRESSANTS Opioid Pain Relievers Anxiolytics (also belong to psychiatric medication category) • codeine (in 222® Tablets, Tylenol® No. 1/2/3/4, Fiorinal® C, Benzodiazepines Codeine Contin, etc.) • heroin • alprazolam (Xanax®) • hydrocodone (Hycodan®, etc.) • chlordiazepoxide (Librium®) • hydromorphone (Dilaudid®) • clonazepam (Rivotril®) • methadone • diazepam (Valium®) • morphine (MS Contin®, M-Eslon®, Kadian®, Statex®, etc.) • flurazepam (Dalmane®) • oxycodone (in Oxycocet®, Percocet®, Percodan®, OxyContin®, etc.) • lorazepam (Ativan®) • pentazocine (Talwin®) • nitrazepam (Mogadon®) • oxazepam ( Serax®) Alcohol • temazepam (Restoril®) Inhalants Barbiturates • gases (e.g. nitrous oxide, “laughing gas”, chloroform, halothane, • butalbital (in Fiorinal®) ether) • secobarbital (Seconal®) • volatile solvents (benzene, toluene, xylene, acetone, naptha and hexane) Buspirone (Buspar®) • nitrites (amyl nitrite, butyl nitrite and cyclohexyl nitrite – also known as “poppers”) Non-Benzodiazepine Hypnotics (also belong to psychiatric medication category) • chloral hydrate • zopiclone (Imovane®) Other • GHB (gamma-hydroxybutyrate) • Rohypnol (flunitrazepam) CENTRAL NERVOUS SYSTEM STIMULANTS Amphetamines Caffeine • dextroamphetamine (Dexadrine®) Methelynedioxyamphetamine (MDA) • methamphetamine (“Crystal meth”) (also has hallucinogenic actions) • methylphenidate (Biphentin®, Concerta®, Ritalin®) • mixed amphetamine salts (Adderall XR®) 3,4-Methelynedioxymethamphetamine (MDMA, Ecstasy) (also has hallucinogenic actions) Cocaine/Crack -
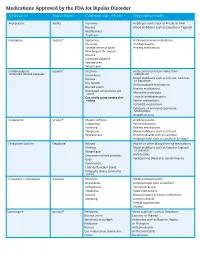
Medications Approved by the FDA for Bipolar Disorder Medication Brand Name Common Side Effects May Interact With
Medications Approved by the FDA for Bipolar Disorder Medication Brand Name Common side effects May interact with Aripiprazole Abilify® Insomnia Antidepressants such as Prozac or Paxil Nausea Mood stabilizers such as Equetro or Tegretol Restlessness Tiredness Asenapine Saphris® Sleepiness Antihypertensive medications Dizziness Antidepressants Strange sense of taste Anxiety medications Numbing of the mouth Nausea Increased appetite Feeling tired Weight gain Carbamazepine Equetro™ Dizziness Birth control pills (can make them extended release capsules Drowsiness ineffective) Mood stabilizers such as Lithium, Lamictal, Nausea or Depakote Dry mouth Anticonvulsant medications Blurred vision Anxiety medications Decreased white blood cell count Macrolide antibiotics Can rarely cause severe skin Tricyclic antidepressants rashes Cancer medications HIV/AIDS medications Cytotoxic or immunosuppressive Medications Grapefruit juice Cariprazine Vraylar® Muscle stiffness Antidepressants Indigestion Pain medications Vomiting Anxiety medications Sleepiness Mood stabilizers such as Lithium Restlessness Anticonvulsants such as Lamictal Antipsychotics such as Latuda or Seroquel Divalproex Sodium Depakote® Nausea Aspirin or other blood thinning medications Shaking Mood stabilizers such as Equetro,Tegretol, Weight gain or Lamictal Decrease in blood platelets Barbiturates Rash Cyclosporine (Neoral or Sandimmune) Pancreatitis Liver dysfunction (rare) Polycystic Ovary Syndrome (rare) Fluoxetine + Olanzapine Symbyax® Dizziness MAOI antidepressants Drowsiness Antipsychotics -

Trimipramine
PATIENT & CAREGIVER EDUCATION Trimipramine This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US Surmontil [DSC] Brand Names: Canada APO-Trimipramine Warning Drugs like this one have raised the chance of suicidal thoughts or actions in children and young adults. The risk may be greater in people who have had these thoughts or actions in the past. All people who take this drug need to be watched closely. Call the doctor right away if signs like low mood (depression), nervousness, restlessness, grouchiness, panic attacks, or changes in mood or actions are new or worse. Call the doctor right away if any thoughts or actions of suicide occur. This drug is not approved for use in children. Talk with the doctor. Trimipramine 1/10 What is this drug used for? It is used to treat low mood (depression). What do I need to tell my doctor BEFORE I take this drug? If you are allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had. If you have had a recent heart attack. If you have taken certain drugs for depression or Parkinson’s disease in the last 14 days. This includes isocarboxazid, phenelzine, tranylcypromine, selegiline, or rasagiline. Very high blood pressure may happen. If you are taking any of these drugs: Linezolid or methylene blue. This is not a list of all drugs or health problems that interact with this drug. -
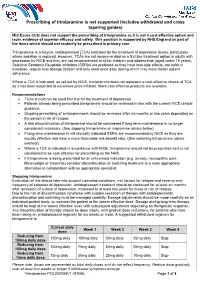
Prescribing of Trimipramine Is Not Supported (Includes Withdrawal and Cross Tapering Guides)
Prescribing of trimipramine is not supported (includes withdrawal and cross tapering guides) Mid Essex CCG does not support the prescribing of trimipramine as it is not a cost-effective option and lacks evidence of superior efficacy and safety. This position is supported by NHS England as part of the items which should not routinely be prescribed in primary care. Trimipramine is a tricyclic antidepressant (TCA) indicated for the treatment of depressive illness, particularly where sedation is required. However, TCAs are not recommended as a first line treatment option in adults with depression by NICE and they are not recommended at all for children and adolescents (aged under 18 years). Selective Serotonin Reuptake Inhibitors (SSRIs) are preferred as they have less side effects, are safer in overdose, require less dosage titration and only need once daily dosing which may mean better patient adherence. Where a TCA is indicated, as set out by NICE, trimipramine does not represent a cost-effective choice of TCA as it has been subjected to excessive price inflation. More cost effective products are available. Recommendations • TCAs should not be used first line for the treatment of depression. • Patients already being prescribed trimipramine should be reviewed in line with the current NICE clinical guidance. • Ongoing prescribing of antidepressants should be reviewed after six months or two years depending on the person’s risk of relapse. • A trial discontinuation of trimipramine should be considered if long-term maintenance is no longer considered necessary. (See stopping trimipramine or imipramine advice below) • If long-term maintenance is still clinically indicated SSRIs are recommended by NICE as they are equally effective and have a more favourable risk-benefit ratio. -

Currently Prescribed Psychotropic Medications
CURRENTLY PRESCRIBED PSYCHOTROPIC MEDICATIONS Schizophrenia Depression Anxiety Disorders 1st generation antipsychotics: Tricyclics: Atarax (hydroxyzine) Haldol (haloperidol), *Anafranil (clomipramine) Ativan (lorazepam) Haldol Decanoate Asendin (amoxapine) BuSpar (buspirone) Loxitane (loxapine) Elavil (amitriptyline) *Inderal (propranolol) Mellaril (thioridazine) Norpramin (desipramine) Keppra (levetiracetam) Navane (thiothixene) Pamelor (nortriptyline) *Klonopin (clonazepam) Prolixin (fluphenazine), Prolixin Sinequan (doxepin) Librium (chlordiazepoxide) Decanoate Spravato (esketamine) Serax (oxazepam) Stelazine (trifluoperazine) Surmontil (trimipramine) Thorazine (chlorpromazine) *Tenormin (atenolol) Tofranil (imipramine) MEDICATIONS PSYCHOTROPIC PRESCRIBED CURRENTLY Trilafon (perphenazine) Tranxene (clorazepate) Vivactil (protriptyline) Valium (diazepam) 2nd generation antipsychotics: Zulresso (brexanolone) Vistaril (hydroxyzine) Abilify (aripiprazole) Aristada (aripiprazole) SSRIs: Xanax (alprazolam) Caplyta (lumateperone) Celexa (citalopram) *Antidepressants, especially SSRIs, are also used in the treatment of anxiety. Clozaril (clozapine) Lexapro (escitalopram) Fanapt (iloperidone) *Luvox (fluvoxamine) Geodon (ziprasidone) Paxil (paroxetine) Stimulants (used in the treatment of ADD/ADHD) Invega (paliperidone) Prozac (fluoxetine) Invega Sustenna Zoloft (sertraline) Adderall (amphetamine and Perseris (Risperidone injectable) dextroamphetamine) Latuda (lurasidone) MAOIs: Azstarys(dexmethylphenidate Rexulti (brexpiprazole) Emsam (selegiline) -

At-A-Glance: Psychotropic Drug Information for Children and Adolescents
At-A-Glance: Psychotropic Drug Information for Children and Adolescents Pediatric Dosage/ Drug Generic FDA Approval Serum Level Name Age/Indication when applicable Warnings and Precautions/Black Box Warnings Combination Antipsychotic/Antidepressant fluoxetine & 18 and older N/A: Pediatric Black Box Warning for fluoxetine/olanzapine olanzapine dosing is currently combination formula (marketed as Symbyax): Usage unavailable or not increased the risk of suicidal thinking and behaviors in applicable for this children and adolescents with major depressive disorder drug. and other psychiatric disorders. Other precautions for fluoxetine/olanzapine combination: Possibly unsafe during lactation. Avoid abrupt withdrawal. Antipsychotic Medications *Precautions which apply to all atypical or second generation antipsychotics (SGA): Neuroleptic Malignant Syndrome/Tardive Dyskinesia/ Hyperglycemia/ Diabetes Mellitus/ Weight Gain/ Akathisia/Dyslipidemia †Precautions which apply to all typical or first generation antipsychotics (FGA): Extrapyramidal symptoms/Tardive Dyskinesia aripiprazole * (SGA) 10 and older for 2-10 mg/kg/day Black Box Warning for aripiprazole: Not approved for bipolar disorder, depression in under age 18. Increased risk of suicidal manic, or mixed thinking and behavior in short-term studies in children episodes; 13 to 17 and adolescents with major depressive disorder and for schizophrenia other psychiatric conditions. and bipolar; 6 to 17 for irritability associated with autistic disorder asenapine* 18 and older N/A Black Box Warning for asenapine: Not approved for dementia-related psychosis. Increased mortality risk for elderly dementia patients due to cardiovascular or infectious events. chlorpromazine† 18 and older 0.25 mg/kg tid Other precautions for chlorpromazine: May alter cardiac (FGA) conduction; sedation; Neuroleptic Malignant Syndrome; weight gain. Use caution with renal disease, seizure disorders, and respiratory disease and in acute illness. -

Antidepressant Medications: U.S. Food and Drug Administration
Antidepressant Medications: U.S. Food and Drug Administration-Approved Indications and Dosages for Use in Adults The therapeutic dosing recommendations for antidepressant medications are based on U.S. Food and Drug Administration (FDA)-approved product labeling. Nevertheless, the dosing regimen is adjusted according to a patient’s individual response to pharmacotherapy. The FDA-approved indications and dosages for the use of antidepressant medications in adults are provided in this table. Some of the antidepressant medications are FDA-approved for the treatment of or as adjunct therapy for Parkinson’s disease. This indication is not discussed in this document because of its very specific focus and individualized treatment regimens. All of the antidepressant medications listed are for oral administration unless otherwise stated. Information on the generic availability of antidepressant medications can be found by searching the Electronic Orange Book at https://www.accessdata.fda.gov/scripts/cder/ob/default.cfm on the FDA website. Generic Medication Indication Initial Dose Maximum Dose Other Information Availability amitriptyline[1] depression Outpatients: Outpatients: Outpatients may be initiated at 50 mg Yes 75 mg per day; 150 mg per day; to 100 mg once a day at bedtime. Dose Hospitalized patients: Hospitalized patients: increases should be made gradually by 100 mg per day 300 mg per day 25 mg to 50 mg as necessary, preferably in the late afternoon or evening. Lower doses are recommended for elderly patients. Take in divided doses. amoxapine[2] depression 50 mg 2 or 3 times a day 400 mg per day; May increase dose to 100 mg 2 to 3 times Yes Hospitalized patients a day by the end of the first week. -

Axert , a New “Triptan”
Rx Details January 1, 2014 Formulary Update The January 2014 Drug Formulary update is included as an attachment. Significant updates include: Oxycodone ER (OxyContin). Quantity Limits for OxyContin have been updated for Commercial Drug Formularies and State Health Care Programs, effective April 1 2014. Quantity limits now allow a maximum of 120mg morphine equivalents per day (MED), similar to other opioid medications. New Daily New Daily mg Strength MED* Quantity Limit limit 10 mg 4 (no change) 40 60 15 mg 4 (no change) 60 90 20 mg 4 (no change) 80 120 30 mg 2 60 90 40 mg 2 80 120 60 mg 0 0 0 80 mg 0 0 0 * MED (morphine equivalents per day) is used to determine dosage levels of different opioids by converting them to morphine equivalents. Additional communications are being sent to affected providers and members. Exceptions allowing higher quantities can be requested if medically necessary. Requests should include a treatment plan, and must include an assessment of the risk of addiction, abuse, and diversion. Tricyclic antidepressants. These tricyclic antidepressants will be limited for elderly members (ages 65 and older) in Commercial and State Health Care Programs, effective April 1 2014. • amitriptyline (Elavil), chlordiazepoxide/ amitriptyline (Limbitrol), and perphenazine/ amitriptyline (Etrafon) • doxepin (Silenor) • imipramine (Tofranil) • trimipramine (Surmontil) • clomipramine (Anafranil). Clomipramine will be approved for obsessive‐compulsive disorder (no additional coverage criteria). These tricyclic antidepressants are anticholinergic, with a high rate of side effects in the elderly. Nortriptyline (Pamelor) and desipramine (Norpramin) remain on formulary for all age groups. Additional communications are being sent to affected providers and members. -

Medications to Avoid Before Skin Testing
PLEASE STOP ANTIHISTAMINES 5 DAYS PRIOR TO NEW PATIENT APPOINTMENTS OR ALLERGY SKIN TESTING *Do not stop asthma medications or any other medications that do not contain antihistamine! **If you have major hives or swelling, do not stop your antihistamines. ***Please call us if you have questions about any of your medications interfering with skin testing. ****Do not use oil, cream or lotion on the back or arms for 24 hours prior to skin testing. COMMON MEDICATIONS CONTAINING ANTIHISTAMINES • Actifed (chlorpheniramine) • Elavil (amitriptyline) • Advil PM, Advil Allergy • Excedrin PM • Alavert/Claritin (loratadine) • Fexofenadine (Allegra) • Allegra (fexofenadine) • Hydroxyzine (Atarax, Vistaril) • Alka Seltzer P.M. • Imipramine (Tofranil) • Amitriptyline (Elavil) • Levocetirizine (Xyzal) • Antivert (Meclizine) • Loratadine (Claritin) • Astelin Nasal Spray (azelastine) • Meclizine (Antivert, Bonine) • Astepro Nasal Spray (azelastine) • Norpramine (desipramine) • Atarax (hydroxyzine) • Nortriptyline (Pamelor) • Azelastine nose spray (Astepro, Astelin) • Nyquil • Benadryl (diphenhydramine) • Nytol • Bonine (meclizine) • Olopatadine (Patanase nasal spray) • Cetirizine (Zyrtec) • Pamelor (nortriptyline) • Chlorpheniramine (Chlor-Trimeton, Actifed, • Patanase Nasal Spray (olopatadine) Tussionex) • PBZ (pyribenzamine) • Chlor-Trimeton (chlorpheniramine) • Pediacare • Clarinex (desloratadine) • Periactin (Cyproheptadine) • Claritin (loratadine) • Phenergan (promethazine) • Clemastine (Tavist) • Promethazine (Phenergan) • Cogentin (for Parkinson's