Medications to Stop Taking Before Allergy Testing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

22-556Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 22-556Orig1s000 MEDICAL REVIEW(S) MEDICAL OFFICER REVIEW Division Of Pulmonary, Allergy, and Rheumatology Products, HFD-570 APPLICATION: NDA 22-556 TRADE NAME: Karbinal ER™ APPLICANT/SPONSOR: Tris Pharma USAN NAME: Carbinoxamine Extended-Release MEDICAL OFFICER: Peter Starke, MD Oral Suspension TEAM LEADER: Theresa Michele, MD CATEGORY: Antihistamine DATE: February 25, 2013 ROUTE: Oral SUBMISSIONS REVIEWED IN THIS DOCUMENT Document Date Submission Date Application/Doc Comments October 4, 2012 October 5, 2012 SD-17 Complete Response submission January 8, 2013 January 9, 2013 SD-20 Response to labeling (formatting) IR RELATED APPLICATIONS Date Application Comments REVIEW SUMMARY: This is clinical review of a Complete Response (CR) to a CR action taken by the Agency on October 7, 2011, for a 505(b)(2) application from Tris Pharma for Carbinoxamine Extended-Release (ER) Oral Suspension, equivalent to 4 mg of carbinoxamine maleate (CM) per 5 mL. The formulation is a sustained release formulation of carbinoxamine maleate suspended in a drug-polistirex resin complex. The proposed Trade Name is Karbinal ER. The application references both the currently available generic immediate-release Carbinoxamine Maleate 4 mg tablets (ANDA 40-442) and oral solution 4 mg/5 mL(ANDA 40-458), marketed under the brand name Palgic and manufactured by Milkart, Inc., and the no-longer-marketed immediate-release innovator products, Clistin 4 mg tablets (NDA 08-915) and 4 mg/5 mL elixir (NDA 08-955), previously marketed by McNeil. McNeil discontinued marketing the Clistin products in the 1990s, and the Orange Book makes the notation that the Clistin products were not discontinued or withdrawn for safety or efficacy reasons. -

XYZAL (Levocetirizine Dihydrochloride)
HIGHLIGHTS OF PRESCRIBING INFORMATION • Patients with end-stage renal impairment at less than 10 mL/min These highlights do not include all the information needed to use XYZAL creatinine clearance or patients undergoing hemodialysis (2.3, 4) safely and effectively. See full prescribing information for XYZAL. • Children 6 to 11 years of age with renal impairment (2.3, 4) ® XYZAL (levocetirizine dihydrochloride) ------------------------WARNINGS AND PRECAUTIONS----------------------- 5 mg tablets 2.5 mg/5 mL (0.5 mg/mL) oral solution • Avoid engaging in hazardous occupations requiring complete mental Initial U.S. Approval: 1995 alertness such as driving or operating machinery when taking XYZAL (5.1). ---------------------------RECENT MAJOR CHANGES--------------------------- • Avoid concurrent use of alcohol or other central nervous system Dosage and Administration (2) 01/2008 depressants with XYZAL (5.1). Dosage and Administration, Adults & Children 12 Years & Older (2.1) 01/2008 ------------------------------ADVERSE REACTIONS------------------------------ Dosage and Administration, Children 6 to 11 Years (2.2) 01/2008 The most common adverse reactions (rate ≥2% and > placebo) were ---------------------------INDICATIONS AND USAGE---------------------------- somnolence, nasopharyngitis, fatigue, dry mouth, and pharyngitis in subjects XYZAL is a histamine H1-receptor antagonist indicated for: 12 years of age and older, and pyrexia, somnolence, cough, and epistaxis in • The relief of symptoms associated with seasonal and perennial allergic -

For Personal Use Only
SedatiWITH ANTIi ® Dowden Health Media CopyrightFor personal use only Initiate the antipsychotic at a reasonable, not overly high dose, then use a nonantipsychotic to help control insomnia, anxiety, and agitation For mass reproduction, content licensing and permissions contact Dowden Health Media. pSYCHIATRY i PSYCHOTICSon edation is a frequent side effect of antipsychot- ics, especially at relatively high doses. Antipsy- S chotics’ sedative effects can reduce agitation in acute psychosis and promote sleep in insomnia, but Manage, don’t long-term sedation may: • interfere with schizophrenia patients’ efforts to go accept adverse to work or school or engage in normal socialization • prevent improvement from psychosocial training, psychiatric rehabilitation, and other treatments. ‘calming’ eff ect This article discusses how to manage acute psycho- sis without oversedation and ways to address persistent sedation and chronic insomnia with less-sedating anti- Del D. Miller, PharmD, MD Professor of psychiatry psychotics or adjunctive medications. University of Iowa Carver College of Medicine Iowa City Neurobiology or psychopharmacology? Many patients experience only mild, transient som- nolence at the beginning of antipsychotic treatment, and most develop some tolerance to the sedating ef- fects with continued administration. Others may have persistent daytime sedation that interferes with nor- mal functioning. Sedation is especially common in elderly patients re- ceiving antipsychotics. Compared with younger patients, older patients receiving -

The Case of Ketamine Allergy
CASE REPORT The Case of Ketamine Allergy William Bylund, MD Department of Emergency Medicine, Naval Medical Center Portsmouth, Portsmouth, Virginia Liam Delahanty, MD Maxwell Cooper, MD Section Editor: Rick A. McPheeters, DO Submission history: Submitted April 3, 2017; Revision received June 29, 2017; Accepted July 11, 2017 Electronically published October 3, 2017 Full text available through open access at http://escholarship.org/uc/uciem_cpcem DOI: 10.5811/cpcem.2017.7.34405 Ketamine is often used for pediatric procedural sedation due to low rates of complications, with allergic reactions being rare. Immediately following intramuscular (IM) ketamine administration, a three-year-old female rapidly developed facial edema and diffuse urticarial rash, with associated wheezing and oxygen desaturation. Symptoms resolved following treatment with epinephrine, dexamethasone and diphenhydramine. This case presents a clinical reaction to ketamine consistent with anaphylaxis due to histamine release, but it is uncertain whether this was immunoglobulin E mediated. This is the only case reported to date of allergic reaction to IM ketamine, without co- administration of other agents. [Clin Pract Cases Emerg Med.2017;1(4):323–325.] INTRODUCTION cardiac monitor, end tidal CO2 (ETCO2) and pulse oximetry Ketamine is a common medication, used in isolation as well (POx), in addition to being placed on two liters nasal cannula as with other agents, for pediatric sedation in the emergency (NC). No intravenous (IV) access was obtained prior to sedation. department (ED). It is often turned to because of its efficacy, ease Seventy milligrams (4.4mg/kg) of ketamine was administered IM of use, and favorable safety profile. Common side effects of into the right thigh. -

FEXOFENADINE HYDROCHLORIDE 180 MG FILM-COATED TABLETS Fexofenadine Hydrochloride
ID1089 MRP _ UK Version: 07 Review Date: 19/02/2020 PACKAGE LEAFLET: INFORMATION FOR THE USER FEXOFENADINE HYDROCHLORIDE 120 MG FILM-COATED TABLETS FEXOFENADINE HYDROCHLORIDE 180 MG FILM-COATED TABLETS Fexofenadine hydrochloride Read all of this leaflet carefully before you start using this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor or pharmacist. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. - If you get any of the side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet, (see section 4). In this leaflet: 1. What Fexofenadine hydrochloride is and what it is used for 2. What you need to know before you take Fexofenadine hydrochloride 3. How to take Fexofenadine hydrochloride 4. Possible side effects of Fexofenadine hydrochloride 5. How to store Fexofenadine hydrochloride 6. Contents of the pack and other information 1. WHAT FEXOFENADINE HYDROCHLORIDE IS AND WHAT IT IS USED FOR FEXOFENADINE HYDROCHLORIDE Contains fexofenadine hydrochloride which is an antihistamine. Only Fexofenadine hydrochloride 120 mg tablets is used in adults and adolescents of 12 years and older to relieve the symptoms that occur with hay fever (seasonal allergic rhinitis) such as sneezing, itchy, running or blocked nose and itchy, red and watery eye). Fexofenadine hydrochloride 180 mg tablets is used in adults and adolescents of 12 years and older to relieve the symptoms that occur with long term allergic skin reactions( chronic idiopathic urticaria) such as itching, swelling and rashes 2. -

New York State Medicaid Drug Utilization Review Board Meeting Agenda
New York State Medicaid Drug Utilization Review Board Meeting Agenda The Drug Utilization Review (DUR) Board will meet on April 27, 2016, from 9:00 a.m. to 4:00 p.m., Meeting Room 6, Concourse, Empire State Plaza, Albany, New York Agenda Items A. Preferred Drug Program (PDP) The DUR Board will review therapeutic classes listed below, as they pertain to the PDP. The DUR Board will review clinical and financial information, to recommend preferred and non-preferred drugs. For therapeutic classes currently subject to the PDP^, the DUR Board will only consider clinical information which is new since the previous review of the therapeutic class and then consider financial information. New clinical information may include a new drug or drug product information, new indications, new safety information or new published clinical trials (comparative evidence is preferred, or placebo controlled when no head-to-head trials are available). Information in abstract form alone, posters, or unpublished data are poor quality evidence for the purpose of re-review and submission is discouraged. Those wishing to submit new clinical information must do so in an electronic format by April 12, 2016 or the Board may not have ample time to review the information. ^ The current preferred and non-preferred status of drugs subject to the Preferred Drug List (PDL) may be viewed at https://newyork.fhsc.com/downloads/providers/NYRx_PDP_PDL.pdf 1. Non-Steroidal Anti-Inflammatory Drugs (NSAIDS) – Prescription (Previous review date: April 22, 2015) Anaprox DS (naproxen -

Cambridgeshire and Peterborough Joint Prescribing Group MEDICINE REVIEW
Cambridgeshire and Peterborough Joint Prescribing Group MEDICINE REVIEW Name of Medicine / Trimipramine (Surmontil®) Class (generic and brand) Licensed indication(s) Treatment of depressive illness, especially where sleep disturbance, anxiety or agitation are presenting symptoms. Sleep disturbance is controlled within 24 hours and true antidepressant action follows within 7 to 10 days. Licensed dose(s) Adults: For depression 50-75 mg/day initially increasing to 150-300 mg/day in divided doses or one dose at night. The maintenance dose is 75-150 mg/day. Elderly: 10-25 mg three times a day initially. The initial dose should be increased with caution under close supervision. Half the normal maintenance dose may be sufficient to produce a satisfactory clinical response. Children: Not recommended. Purpose of Document To review information currently available on this class of medicines, give guidance on potential use and assign a prescribing classification http://www.cambsphn.nhs.uk/CJPG/CurrentDrugClassificationTable.aspx Annual cost (FP10) 10mg three times daily: £6,991 25mg three times daily: £7,819 150mg daily: £7,410 300mg daily: £14,820 Alternative Treatment Options within Class Tricyclic Annual Cost CPCCG Formulary Classification Antidepressant (FP10) Amitriptyline (75mg) Formulary £36 Lofepramine (140mg) Formulary £146 Imipramine (75mg) Non-formulary £37 Clomipramine (75mg) Non-formulary £63 Trimipramine (75mg). TBC £7,819 Nortriptyline (75mg) Not Recommended (pain) £276 Doxepin (150mg) TBC £6,006 Dosulepin (75mg) Not Recommended (NICE DO NOT DO) £19 Dosages are based on possible maintenance dose and are not equivalent between medications Recommendation It is recommended to Cambridgeshire and Peterborough CCG JPG members and through them to local NHS organisations that the arrangements for use of trimipramine are in line with restrictions agreed locally for drugs designated as NOT RECOMMENDED:. -

Comparative Effects of Cimetidine and Famotidine on the Vagally Stimulated Acid Secretion in the Isolated Mouse Whole Stomach
Comparative Effects of Cimetidine and Famotidine on the Vagally Stimulated Acid Secretion in the Isolated Mouse Whole Stomach Kazuo Watanabe1, Shingo Yano1, Masayuki Yamamoto1 and Shoko Kanaoka2 1Laboratory of Chemical Pharmacology, Department of Drug Evaluation and Toxicological Sciences, Faculty of Pharmaceutical Sciences, Chiba University, 1-33, Yayoi-cho, Inage-ku, Chiba 263, Japan 2Research Institute for Wakan-Yaku, Toyama Medical and Pharmaceutical University, Toyama 930-01, Japan Received July 15, 1992 Accepted December 10, 1992 ABSTRACT-We investigated the effects of cimetidine and famotidine on the acid secretory response to elec trical vagal stimulation, bethanechol and histamine in the isolated mouse whole stomach preparation. The acid secretion elicited by electrical vagal stimulation at the position of the esophagus (10 Hz, 0.3 msec, 10 V for 5 min) was reproducible by repeated stimulation in each preparation, and it was abolished by tetrodo toxin, atropine and hexamethonium. This vagally stimulated acid secretion was abolished by cimetidine (3 mM), while it was only partly inhibited by famotidine (10-100 ƒÊM). Histamine (100 ƒÊM)-induced acid secre tion was inhibited by cimetidine and famotidine, and the doses of these drugs required for complete inhibi tion were 3 mM and 10 ƒÊM, respectively. In contrast, bethanechol (10 ƒÊM)-induced acid secretion was slight ly reduced by famotidine (1-100 ƒÊM), but markedly reduced by cimetidine (3 mM). In the guinea pig ileum, millimolar concentrations of cimetidine and famotidine shifted the dose-response curve of the contractile response to acetylcholine rightward. These findings suggest that the inhibitory effect of cimetidine on the vagally stimulated or bethanechol-induced acid secretion is elicited at least partly through mechanisms different from H2-antagonism. -

Diphenhydramine Dosage Sheet Concord Pediatrics, P.A
Diphenhydramine Dosage Sheet Concord Pediatrics, P.A. (603) 224-1929 BRAND NAMES: Benadryl INDICATIONS: Treatment of allergic reactions, nasal allergies, hives and itching. FREQUENCY: Repeat every 6 hours as needed. Don't give more than 4 times a day. DOSAGE: Determine by using the table below. Please speak with a medical provider before giving Diphenhydramine to a child under 1 year old. Child’s Dose in mg Children’s Liquid Chewable/Fastmelts Tabs/Caps/Gels Weight (lb) (12.5mg/ 5mL) 12.5mg tablets 25mg tablets *12-16 lbs 6.25 mg 2.5 mL *17-19 lbs 9.375 mg 3.75 mL *20-24 lbs 10 mg 4 mL 25-37 lbs 12.5 mg 5 mL 1 tab 38-49 lbs 18.75 mg 7.5 mL 1.5 tabs 50-69 lbs 25 mg 10 mL 2 tabs 1 tab 70-99 lbs 37.5 mg 15 mL 3 tabs 1.5 tabs >100 lbs 50 mg 20 mL 4 tabs 2 tabs Table Notes: *AGE LIMIT: For allergies, don't use under 1 year of age (Reason: it's a sedative). For colds, not recommended at any age (Reason: no proven benefits) and should be avoided if under 4 years old. Avoid multi-ingredient products in children under 6 years of age (Reason: FDA recommendations 10/2008). MEASURING the DOSAGE: Syringes and droppers are more accurate than teaspoons. If possible, use the syringe or dropper that comes with the medicine. If not, medicine syringes are available at pharmacies. Regular spoons are not reliable. ADULT DOSAGE: 50 mg Why use Diphenhydramine? Antihistamines can be used to treat your child’s runny nose, itchy eyes, and sneezing due to allergies. -

A Comparative Evaluation of Lafutidine and 2 Rabeprazole in The
1 *Original research paper 2 A comparative evaluation of Lafutidine and 3 Rabeprazole in the treatment of gastritis and 4 peptic ulcer: A double-blind, randomized study 5 in Indian patients. 6 Dr. Sanjay Kumar 1, Dr. Bhupesh Dewan 2*, Deepashri Shah 2 7 8 1Global Liver and Gastroenterology Centre, Bhopal, India 2 9 Medical Department, Zuventus Healthcare Ltd., Mumbai, India 10 11 . 12 ABSTRACT 13 Aims: To assess the efficacy of lafutidine therapy versus rabeprazole in Indian patients with endoscopically and histologically proven gastritis and peptic ulcer. Study design: A double blind, double dummy, randomized, comparative study. Place and Duration of Study: Global Liver and Gastroenterology Centre, Bhopal, India, between March 2010 and October 2010. Methodology: A total of 100 patients were enrolled, including 50 with endoscopically and histologically proven gastritis and other 50 with peptic ulcer (over 5 mm in diameter). Each group was randomized to receive either lafutidine or rabeprazole tablet and their corresponding competitor placebo dummy tablet, for a period of 4 weeks. Gastritis/ulcer cure rates confirmed by endoscopic histology, symptom response and Helicobacter pylori (H. Pylori) eradication were compared among the two drugs Results: Complete cure of gastritis was observed in all the patients (100%) treated with lafutidine and 95.24% [20/21; 95% CI: 76.18 to 99.88%] patients treated with rabeprazole. Complete cure of ulcer was observed in 72.0% (18/25, 95% CI = 50.61 to 87.93%) and 79.16% (19/24, 95% CI = 57.85 to 92.87%) patients treated with lafutidine and rabeprazole respectively. There was no significant difference in gastritis/ulcer cure rate and symptom response rate between the two treatment groups at the end of the study. -
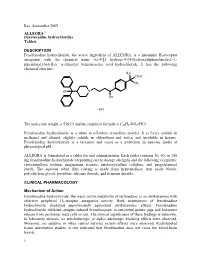
Fexofenadine Hydrochloride) Tablets
Rev. September 2005 ALLEGRA® (fexofenadine hydrochloride) Tablets DESCRIPTION Fexofenadine hydrochloride, the active ingredient of ALLEGRA, is a histamine H1-receptor antagonist with the chemical name (±)-4-[1 hydroxy-4-[4-(hydroxydiphenylmethyl)-1- piperidinyl]-butyl]-α, α-dimethyl benzeneacetic acid hydrochloride. It has the following chemical structure: The molecular weight is 538.13 and the empirical formula is C32H39NO4•HCl. Fexofenadine hydrochloride is a white to off-white crystalline powder. It is freely soluble in methanol and ethanol, slightly soluble in chloroform and water, and insoluble in hexane. Fexofenadine hydrochloride is a racemate and exists as a zwitterion in aqueous media at physiological pH. ALLEGRA is formulated as a tablet for oral administration. Each tablet contains 30, 60, or 180 mg fexofenadine hydrochloride (depending on the dosage strength) and the following excipients: croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and pregelatinized starch. The aqueous tablet film coating is made from hypromellose, iron oxide blends, polyethylene glycol, povidone, silicone dioxide, and titanium dioxide. CLINICAL PHARMACOLOGY Mechanism of Action Fexofenadine hydrochloride, the major active metabolite of terfenadine, is an antihistamine with selective peripheral H1-receptor antagonist activity. Both enantiomers of fexofenadine hydrochloride displayed approximately equipotent antihistaminic effects. Fexofenadine hydrochloride inhibited antigen-induced bronchospasm in sensitized guinea pigs and histamine -

MEDICATIONS to AVOID PRIOR to ALLERGY SKIN TESTING Allergy
MEDICATIONS TO AVOID PRIOR TO ALLERGY SKIN TESTING Allergy testing requires the ‘histamine response’ in order to be accurate and reliable. There are many types of antihistamines. Antihistamines are found in many different medicines, either as a single drug or mixed with a combination of chemicals. Please review all medicines you take (including Over-The-Counter) in order to make your allergy testing appointment most efficient and accurate. Generic names are in all lower case, trade names Capitalized. Oral antihistamines to be stopped 3 (THREE) days prior to your appointment: - brompheniramine (Actifed, Atrohist, Dimetapp, Drixoral) - cetirizine (Zyrtec, Zyrtec D) - chlopheniramine (Chlortrimeton, Deconamine, Kronofed A, Novafed A, Rynatan, Tussinex) - clemastine (Tavist, Antihist) - cyproheptadine (Periactin) - diphenhydramine (Benadryl, Allernix, Nytol) - doxylamine (Bendectin, Nyquil) - hydroxyzine (Atarax, Marax, Vistaril) - levocetirizine (Xyzal) - promethazine (Phenergan) Oral antihistamines to be stopped 7 (SEVEN) days prior to your appointment: - desloratadine (Clarinex) - fexofenadine (Allegra, Allegra D) - loratadine (Claritin, Claritin D, Alavert) Nose spray and eye drop antihistamines to stop 5 (FIVE) days prior to your appointment: - azelastine (Astelin, Astepro, Dymista, Optivar) - bepotastine (Bepreve) - ketotifen (Zaditor, Alaway) - olapatadine (Pataday, Patanase) - pheniramine (Visine A, Naphcon A) – OK to stop for 2 days Antacid medications (different type of antihistamine) to stop 3 (THREE) days prior to your appointment: - cimetidine (Tagamet) - famotidine (Pepcid) - ranitidine (Zantac) Note: Antihistamines are found in many over the counter medications, including Tylenol Allergy, Actifed Cold and Allergy, Alka-Seltzer Plus Cold with Cough Formula, and many others. Make sure you read and check the ingredients carefully and stop those containing antihistamines at least 3 (THREE) days prior to the appointment.