Review Memorandum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Educational Commentary – Testing for Amphetamines and Related Compounds
EDUCATIONAL COMMENTARY – TESTING FOR AMPHETAMINES AND RELATED COMPOUNDS Learning Outcomes Upon completion of this exercise, participants will be able to: • list amphetamine-like compounds that may be detected by immunoassay screening tests for amphetamines. • describe the 3 types of amphetamine immunoassays based on their antibody specificity. • discuss common causes of false-positive results when testing for amphetamines. Correct interpretation of testing results for amphetamines and related compounds is dependent on many factors including an understanding of the nomenclature, structure, and metabolism of these compounds. The class of phenethylamine compounds having varying degrees of sympathomimetic activity include amphetamine, methamphetamine, and many other compounds known by several names including amphetamines (as a group), designer drugs often grouped together as ecstasy [3,4- methylenedioxymethamphetamine (MDMA); 3,4-methylenedioxyamphetamine (MDA), and 3,4- methylenedioxy-N-ethylamphetamine (MDEA)], and sympathomimetic amines including ephedrine, pseudoephedrine, and phenylpropanolamine found in over-the-counter (OTC) medications. Amphetamine, a primary amine, and methamphetamine, a secondary amine, have a stereogenic center and have enantiomers or optical isomers designated d (or +) for dextrorotatory and l (or -) for levorotatory. In general, the d isomers are the more physiologically active compounds, but pharmaceutical preparations may consist of either isomer or a mixture of both isomers. When the mixture contains equal concentrations of the 2 enantiomers, it is known as a racemic mixture. The existence of enantiomers for amphetamines creates analytical and interpretative problems. Immunoassay Screening The primary screening methods for detecting amphetamines are immunoassays. The structural similarity to amphetamine or methamphetamine of the compounds previously mentioned makes it difficult to produce antibodies specific for amphetamine, methamphetamine, or both. -

Drug Formulary Medi-Cal/Healthy Families Drug Formulary • 2014
2014 Drug Formulary Medi-Cal/Healthy Families Drug Formulary • 2014 Medi-Cal/Healthy Families Drug Formulary • 2014 TABLE OF CONTENTS MOLINA HEALTHCARE MEDI-CAL/HEALTHY FAMILIES DRUG FORMULARY ....................................................................................4 PRESCRIPTION CLAIMS PROCESSOR .........................................................5 USING THE MOLINA MEDI-CAL/HEALTHY FAMILIES DRUG FORMULARY ....................................................................................6 CLINICAL CONSIDERATIONS .........................................................................6 INDIVIDUAL PRESCRIPTIONS ........................................................................6 GENERIC MEDICATIONS .................................................................................7 PRIOR AUTHORIZATION REQUEST PROCEDURE ......................................7 STEP THERAPY PROCEDURE .......................................................................7 PRESCRIPTION QUANTITY .............................................................................7 URGENT AND AFTER-HOURS MEDICATION POLICY ..................................8 TELEPHONE PRESCRIPTIONS.......................................................................8 DRUG FORMULARY .........................................................................................9 Chapter 1 ANALGESICS ..............................................................................9 Chapter 2 ANTIDIABETIC AGENTS ..........................................................12 Chapter -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2020 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

Acetaminophen, Isometheptene, and Dichloralphenazone
PATIENT & CAREGIVER EDUCATION Acetaminophen, Isometheptene, and Dichloralphenazone This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US Nodolor [DSC] Warning This drug has acetaminophen in it. Liver problems have happened with the use of acetaminophen. Sometimes, this has led to a liver transplant or death. Most of the time, liver problems happened in people taking more than 4,000 mg (milligrams) of acetaminophen in a day. People were also often taking more than 1 drug that had acetaminophen. Avoid drinking alcohol while taking this drug. What is this drug used for? It is used to treat headaches. Acetaminophen, Isometheptene, and Dichloralphenazone 1/8 What do I need to tell my doctor BEFORE I take this drug? If you have an allergy to acetaminophen, isometheptene, dichloralphenazone, or any other part of this drug. If you are allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had. If you have any of these health problems: Glaucoma, heart disease, high blood pressure, liver disease, or poor kidney function. If you have blood vessel problems, including in the heart or brain. If you have had a recent heart attack or stroke. If you have taken certain drugs for depression or Parkinson’s disease in the last 14 days. This includes isocarboxazid, phenelzine, tranylcypromine, selegiline, or rasagiline. Very high blood pressure may happen. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

PQA Measure Specifications ______
PQA Measure Specifications ______________________________________________________________________________ Table DDI-A: Target Medications and Precipitant Medications Table DDI-A Category Target Drug or Drug Class (Step 1) Precipitant Drug or Drug Class (Step 2) A Benzodiazepines: alprazolam, midazolam, triazolam Azole antifungal agents: ketoconazole, itraconazole, fluconazole, posaconazole, voriconazole B carbamazepine Clarithromycin, erythromycin, telithromycin C cyclosporine Rifamycins: rifampin, rifabutin, rifapentine D digoxin clarithromycin, erythromycin, azithromycin, telithromycin E Ergot alkaloids: ergotamine, dihydroergotamine clarithromycin, erythromycin, telithromycin F Estrogen/progestin oral contraceptives: Rifamycins: rifampin, rifabutin, rifapentine desogestrel-ethinyl estradiol, drospirenone-ethinyl estradiol, estradiol valerate-dienogest, ethinyl estradiol-ethynodiol, ethinyl estradiol-levonorgestrel, ethinyl estradiol-norethindrone, ethinyl estradiol- norgestimate, ethinyl estradiol-norgestrel, mestranol- norethindrone G MAO Inhibitors: isocarboxazid, linezolid, phenelzine, Sympathomimetics: amphetamines, atomoxetine, rasagiline, selegiline, tranylcypromine, sibutramine benzphetamine, dextroamphetamine, diethylpropion, isometheptene, methamphetamine, methylphenidate, phendimetrazine, phentermine, phenylephrine, pseudoephedrine, tapentadol, dexmethylphenidate, lisdexamfetamine Serotonergic Agents: buspirone, citalopram, cyclobenzaprine, desvenlafaxine, dextromethorphan, duloxetine, escitalopram, fluoxetine, fluvoxamine, -
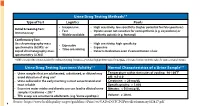
Urine Drug Testing Methods3-5 Urine Drug Testing Specimen Validity3-4
Urine Drug Testing Methods3-5 Type of Test Logistics Pearls • Inexpensive • High sensitivity, low specificity (higher potential for false positives) Initial Screening Test: • Fast • Opiate screen not sensitive for semisynthetic (e.g. oxycodone) or Immunoassay • Widely available synthetic opioids (e.g. fentanyl) Confirmatory Test: Gas chromatography-mass • High sensitivity, high specificity • Expensive spectrometry (GCMS)+ or • Expensive • Time consuming Liquid chromatography-mass • Detects medication even if concentration is low spectrometry (LCMS) + GCMS is considered the criterion standard for confirmatory testing; Immunoassay tests have high predictive values for marijuana and cocaine, but lower predictive values for opiates and amphetamines Urine Drug Testing Specimen Validity3-4 Normal Characteristics of a Urine Sample3-5 • Urine samples that are adulterated, substituted, or diluted may Temperature within 4 minutes of voiding: 90-100⁰F avoid detection of drug use4 pH: 4.5-8.0 • Urine collected in the early morning is most concentrated and Creatinine: > 20 mg/dL most reliable Specific gravity: > 1.003 • Excessive water intake and diuretic use can lead to diluted urine Nitrates: < 500 mcg/dL samples (Creatinine < 20) 3-4 • THC assays are sensitive to adulterants (e.g. Visine eyedrops) Volume: ≥ 30 mL 4 Source: https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPGProviderSummary022817.pdf Urine Drug Testing (UDT) Federal Work Place Cut Off Values3-9 Initial Drug Test Level Confirmatory Drug Test Detection Period After Confirmatory -

Appendix-2Final.Pdf 663.7 KB
North West ‘Through the Gate Substance Misuse Services’ Drug Testing Project Appendix 2 – Analytical methodologies Overview Urine samples were analysed using three methodologies. The first methodology (General Screen) was designed to cover a wide range of analytes (drugs) and was used for all analytes other than the synthetic cannabinoid receptor agonists (SCRAs). The analyte coverage included a broad range of commonly prescribed drugs including over the counter medications, commonly misused drugs and metabolites of many of the compounds too. This approach provided a very powerful drug screening tool to investigate drug use/misuse before and whilst in prison. The second methodology (SCRA Screen) was specifically designed for SCRAs and targets only those compounds. This was a very sensitive methodology with a method capability of sub 100pg/ml for over 600 SCRAs and their metabolites. Both methodologies utilised full scan high resolution accurate mass LCMS technologies that allowed a non-targeted approach to data acquisition and the ability to retrospectively review data. The non-targeted approach to data acquisition effectively means that the analyte coverage of the data acquisition was unlimited. The only limiting factors were related to the chemical nature of the analyte being looked for. The analyte must extract in the sample preparation process; it must chromatograph and it must ionise under the conditions used by the mass spectrometer interface. The final limiting factor was presence in the data processing database. The subsequent study of negative MDT samples across the North West and London and the South East used a GCMS methodology for anabolic steroids in addition to the General and SCRA screens. -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine -

No 1223/2009 of the EUROPEAN PARLIAMENT and of the COUNCIL of 30 November 2009 on Cosmetic Products (Recast) (Text with EEA Relevance) (OJ L 342, 22.12.2009, P
02009R1223 — EN — 03.12.2020 — 025.001 — 1 This text is meant purely as a documentation tool and has no legal effect. The Union's institutions do not assume any liability for its contents. The authentic versions of the relevant acts, including their preambles, are those published in the Official Journal of the European Union and available in EUR-Lex. Those official texts are directly accessible through the links embedded in this document ►B REGULATION (EC) No 1223/2009 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 30 November 2009 on cosmetic products (recast) (Text with EEA relevance) (OJ L 342, 22.12.2009, p. 59) Amended by: Official Journal No page date ►M1 Commission Regulation (EU) No 344/2013 of 4 April 2013 L 114 1 25.4.2013 ►M2 Commission Regulation (EU) No 483/2013 of 24 May 2013 L 139 8 25.5.2013 ►M3 Commission Regulation (EU) No 658/2013 of 10 July 2013 L 190 38 11.7.2013 ►M4 Commission Regulation (EU) No 1197/2013 of 25 November 2013 L 315 34 26.11.2013 ►M5 Commission Regulation (EU) No 358/2014 of 9 April 2014 L 107 5 10.4.2014 ►M6 Commission Regulation (EU) No 866/2014 of 8 August 2014 L 238 3 9.8.2014 ►M7 Commission Regulation (EU) No 1003/2014 of 18 September 2014 L 282 1 26.9.2014 ►M8 Commission Regulation (EU) No 1004/2014 of 18 September 2014 L 282 5 26.9.2014 ►M9 Commission Regulation (EU) 2015/1190 of 20 July 2015 L 193 115 21.7.2015 ►M10 Commission Regulation (EU) 2015/1298 of 28 July 2015 L 199 22 29.7.2015 ►M11 Commission Regulation (EU) 2016/314 of 4 March 2016 L 60 59 5.3.2016 ►M12 Commission Regulation (EU) -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Pharmacology of Stimulants Prohibited by the World Anti-Doping Agency (WADA)
British Journal of Pharmacology (2008) 154, 606–622 & 2008 Nature Publishing Group All rights reserved 0007– 1188/08 $30.00 www.brjpharmacol.org REVIEW Pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA) JR Docherty Department of Physiology, Royal College of Surgeons in Ireland, Dublin, Ireland This review examines the pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA). Stimulants that increase alertness/reduce fatigue or activate the cardiovascular system can include drugs like ephedrine available in many over- the-counter medicines. Others such as amphetamines, cocaine and hallucinogenic drugs, available on prescription or illegally, can modify mood. A total of 62 stimulants (61 chemical entities) are listed in the WADA List, prohibited in competition. Athletes may have stimulants in their body for one of three main reasons: inadvertent consumption in a propriety medicine; deliberate consumption for misuse as a recreational drug and deliberate consumption to enhance performance. The majority of stimulants on the list act on the monoaminergic systems: adrenergic (sympathetic, transmitter noradrenaline), dopaminergic (transmitter dopamine) and serotonergic (transmitter serotonin, 5-HT). Sympathomimetic describes agents, which mimic sympathetic responses, and dopaminomimetic and serotoninomimetic can be used to describe actions on the dopamine and serotonin systems. However, many agents act to mimic more than one of these monoamines, so that a collective term of monoaminomimetic may be useful. Monoaminomimietic actions of stimulants can include blockade of re-uptake of neurotransmitter, indirect release of neurotransmitter, direct activation of monoaminergic receptors. Many of the stimulants are amphetamines or amphetamine derivatives, including agents with abuse potential as recreational drugs.