Acetaminophen, Isometheptene, and Dichloralphenazone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Educational Commentary – Testing for Amphetamines and Related Compounds
EDUCATIONAL COMMENTARY – TESTING FOR AMPHETAMINES AND RELATED COMPOUNDS Learning Outcomes Upon completion of this exercise, participants will be able to: • list amphetamine-like compounds that may be detected by immunoassay screening tests for amphetamines. • describe the 3 types of amphetamine immunoassays based on their antibody specificity. • discuss common causes of false-positive results when testing for amphetamines. Correct interpretation of testing results for amphetamines and related compounds is dependent on many factors including an understanding of the nomenclature, structure, and metabolism of these compounds. The class of phenethylamine compounds having varying degrees of sympathomimetic activity include amphetamine, methamphetamine, and many other compounds known by several names including amphetamines (as a group), designer drugs often grouped together as ecstasy [3,4- methylenedioxymethamphetamine (MDMA); 3,4-methylenedioxyamphetamine (MDA), and 3,4- methylenedioxy-N-ethylamphetamine (MDEA)], and sympathomimetic amines including ephedrine, pseudoephedrine, and phenylpropanolamine found in over-the-counter (OTC) medications. Amphetamine, a primary amine, and methamphetamine, a secondary amine, have a stereogenic center and have enantiomers or optical isomers designated d (or +) for dextrorotatory and l (or -) for levorotatory. In general, the d isomers are the more physiologically active compounds, but pharmaceutical preparations may consist of either isomer or a mixture of both isomers. When the mixture contains equal concentrations of the 2 enantiomers, it is known as a racemic mixture. The existence of enantiomers for amphetamines creates analytical and interpretative problems. Immunoassay Screening The primary screening methods for detecting amphetamines are immunoassays. The structural similarity to amphetamine or methamphetamine of the compounds previously mentioned makes it difficult to produce antibodies specific for amphetamine, methamphetamine, or both. -

Drug Formulary Medi-Cal/Healthy Families Drug Formulary • 2014
2014 Drug Formulary Medi-Cal/Healthy Families Drug Formulary • 2014 Medi-Cal/Healthy Families Drug Formulary • 2014 TABLE OF CONTENTS MOLINA HEALTHCARE MEDI-CAL/HEALTHY FAMILIES DRUG FORMULARY ....................................................................................4 PRESCRIPTION CLAIMS PROCESSOR .........................................................5 USING THE MOLINA MEDI-CAL/HEALTHY FAMILIES DRUG FORMULARY ....................................................................................6 CLINICAL CONSIDERATIONS .........................................................................6 INDIVIDUAL PRESCRIPTIONS ........................................................................6 GENERIC MEDICATIONS .................................................................................7 PRIOR AUTHORIZATION REQUEST PROCEDURE ......................................7 STEP THERAPY PROCEDURE .......................................................................7 PRESCRIPTION QUANTITY .............................................................................7 URGENT AND AFTER-HOURS MEDICATION POLICY ..................................8 TELEPHONE PRESCRIPTIONS.......................................................................8 DRUG FORMULARY .........................................................................................9 Chapter 1 ANALGESICS ..............................................................................9 Chapter 2 ANTIDIABETIC AGENTS ..........................................................12 Chapter -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2020 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

Review Memorandum
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k062575 B. Purpose for Submission: New device C. Measurand: Amphetamine, Barbiturates, Benzodiazepines, Cocaine, Methamphetamine, Phencyclidine, Methadone, Opiates, THC, and Tricyclic Antidepressants. D. Type of Test: Qualitative immunoassay E. Applicant: Princeton BioMeditech Corporation F. Proprietary and Established Names: AccuSign RC DOA10 (MET/OPI/COC/THC/PCP/BZO/BAR/MTD/TCA/AMP) StatusFirst DOA10 (MET/OPI/COC/THC/PCP/BZO/BAR/MTD/TCA/AMP) G. Regulatory Information: Product Code Classification Regulation Section Panel LAG II 862.3610 91 (Tox) Methamphetamine test system DJG II 862.3650, Enzyme 91 (Tox) Immunoassay, Opiates DIO II 862.3250, Enzyme 91 (Tox) Immunoassay, Cocaine and Cocaine Metabolites 1 Product Code Classification Regulation Section Panel DKE II 862.3870 91 (Tox) Cannabinoid test system LCM Unclassified (510k 862.3100 Enzyme 91 (Tox) required) immunoassay, Phencyclidine DKZ II 862.3100 91 (Tox) Amphetamine test system JXM II 862.3170 Enzyme 91 (Tox) immunoassay, Barbiturate DIS II 862.3150 91 (Tox) Barbiturate test system DJR II 862.3620 91 (Tox) Methadone test system LFI II 862.3910 Tricyclic 91 (Tox) antidepressant drugs test system H. Intended Use: 1. Intended use(s): See indications for use below. 2. Indication(s) for use: Immunoassay for the qualitative detection of methamphetamine, opiates, cocaine metabolite, THC metabolite, phencyclidine, benzodiazepines, barbiturates, tricyclic antidepressants, methadone, and amphetamine in human urine to assist in screening of drug of abuse samples. The detecting cut-off concentrations are as follows: MET D-Methamphetamine 1000 ng/mL OPI Morphine 300 ng/mL COC Benzoylecgonine 300 ng/mL THC 11-nor-Δ9-9-carboxylic acid 50 ng/mL PCP Phencyclidine 25 ng/mL Benzodiazepine Oxazepam 300 ng/mL Barbiturate Secobarbital 300 ng/mL Methadone Methadone 300 ng/mL TCA Nortriptyline 1000 ng/mL AMP D-Amphetamine 1000 ng/mL 2 This assay provides only a preliminary analytical test result. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Medi-Cal Managed Care Formulary
2021 California Medi-Cal Managed Care Formulary (List of Covered Drugs) PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER WHEN YOU PARTICIPATE IN A MEDI-CAL MANAGED CARE PLAN OFFERED BY KAISER PERMANENTE. This prescription drug formulary was updated on 09/01/2021 and is effective as of September 07, 2021. This formulary document is subject to change and may vary depending on your health plan. For more recent information or questions about which drug formulary applies to your plan, visit kp.org/formulary or call our Member Service Contact Center 24 hours a day, seven days a week (closed holidays). 1-800-464-4000 English (and over 150 languages), 1-800-788-0616 Spanish, 1-800-757-7585 Chinese dialects and 711 TTY for the deaf or hard of hearing. What is the Kaiser Permanente California Medi-Cal Managed Care Formulary? The California Medi-Cal Managed Care Formulary is a list of covered drugs chosen by a group of Kaiser Permanente doctors and pharmacists known as the Pharmacy and Therapeutics Committee. The Committee meets regularly to evaluate and select drugs that are safe and effective for our members. This Formulary meets the requirements outlined under state law, regulations, and guidance for Medi-Cal Managed Care plans. What drugs are covered? Kaiser Permanente covers brand, generic and specialty drugs listed on the California Medi-Cal Managed Care Formulary as long as the drug is medically necessary, the prescription is filled at a Kaiser Permanente, or an affiliated pharmacy, and other coverage rules are followed. If you are prescribed a drug on the California Medi-Cal Managed Care Formulary, that drug will be covered under the terms of your drug benefit. -

124.210 Schedule IV — Substances Included. 1
1 CONTROLLED SUBSTANCES, §124.210 124.210 Schedule IV — substances included. 1. Schedule IV shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. 2. Narcotic drugs. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation containing any of the following narcotic drugs, or their salts calculated as the free anhydrous base or alkaloid, in limited quantities as set forth below: a. Not more than one milligram of difenoxin and not less than twenty-five micrograms of atropine sulfate per dosage unit. b. Dextropropoxyphene (alpha-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2- propionoxybutane). c. 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol, its salts, optical and geometric isomers and salts of these isomers (including tramadol). 3. Depressants. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation which contains any quantity of the following substances, including its salts, isomers, and salts of isomers whenever the existence of such salts, isomers, and salts of isomers is possible within the specific chemical designation: a. Alprazolam. b. Barbital. c. Bromazepam. d. Camazepam. e. Carisoprodol. f. Chloral betaine. g. Chloral hydrate. h. Chlordiazepoxide. i. Clobazam. j. Clonazepam. k. Clorazepate. l. Clotiazepam. m. Cloxazolam. n. Delorazepam. o. Diazepam. p. Dichloralphenazone. q. Estazolam. r. Ethchlorvynol. s. Ethinamate. t. Ethyl Loflazepate. u. Fludiazepam. v. Flunitrazepam. w. Flurazepam. x. Halazepam. y. Haloxazolam. z. Ketazolam. aa. Loprazolam. ab. Lorazepam. ac. Lormetazepam. ad. Mebutamate. ae. Medazepam. af. Meprobamate. ag. Methohexital. ah. Methylphenobarbital (mephobarbital). -

PQA Measure Specifications ______
PQA Measure Specifications ______________________________________________________________________________ Table DDI-A: Target Medications and Precipitant Medications Table DDI-A Category Target Drug or Drug Class (Step 1) Precipitant Drug or Drug Class (Step 2) A Benzodiazepines: alprazolam, midazolam, triazolam Azole antifungal agents: ketoconazole, itraconazole, fluconazole, posaconazole, voriconazole B carbamazepine Clarithromycin, erythromycin, telithromycin C cyclosporine Rifamycins: rifampin, rifabutin, rifapentine D digoxin clarithromycin, erythromycin, azithromycin, telithromycin E Ergot alkaloids: ergotamine, dihydroergotamine clarithromycin, erythromycin, telithromycin F Estrogen/progestin oral contraceptives: Rifamycins: rifampin, rifabutin, rifapentine desogestrel-ethinyl estradiol, drospirenone-ethinyl estradiol, estradiol valerate-dienogest, ethinyl estradiol-ethynodiol, ethinyl estradiol-levonorgestrel, ethinyl estradiol-norethindrone, ethinyl estradiol- norgestimate, ethinyl estradiol-norgestrel, mestranol- norethindrone G MAO Inhibitors: isocarboxazid, linezolid, phenelzine, Sympathomimetics: amphetamines, atomoxetine, rasagiline, selegiline, tranylcypromine, sibutramine benzphetamine, dextroamphetamine, diethylpropion, isometheptene, methamphetamine, methylphenidate, phendimetrazine, phentermine, phenylephrine, pseudoephedrine, tapentadol, dexmethylphenidate, lisdexamfetamine Serotonergic Agents: buspirone, citalopram, cyclobenzaprine, desvenlafaxine, dextromethorphan, duloxetine, escitalopram, fluoxetine, fluvoxamine, -
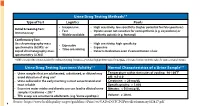
Urine Drug Testing Methods3-5 Urine Drug Testing Specimen Validity3-4
Urine Drug Testing Methods3-5 Type of Test Logistics Pearls • Inexpensive • High sensitivity, low specificity (higher potential for false positives) Initial Screening Test: • Fast • Opiate screen not sensitive for semisynthetic (e.g. oxycodone) or Immunoassay • Widely available synthetic opioids (e.g. fentanyl) Confirmatory Test: Gas chromatography-mass • High sensitivity, high specificity • Expensive spectrometry (GCMS)+ or • Expensive • Time consuming Liquid chromatography-mass • Detects medication even if concentration is low spectrometry (LCMS) + GCMS is considered the criterion standard for confirmatory testing; Immunoassay tests have high predictive values for marijuana and cocaine, but lower predictive values for opiates and amphetamines Urine Drug Testing Specimen Validity3-4 Normal Characteristics of a Urine Sample3-5 • Urine samples that are adulterated, substituted, or diluted may Temperature within 4 minutes of voiding: 90-100⁰F avoid detection of drug use4 pH: 4.5-8.0 • Urine collected in the early morning is most concentrated and Creatinine: > 20 mg/dL most reliable Specific gravity: > 1.003 • Excessive water intake and diuretic use can lead to diluted urine Nitrates: < 500 mcg/dL samples (Creatinine < 20) 3-4 • THC assays are sensitive to adulterants (e.g. Visine eyedrops) Volume: ≥ 30 mL 4 Source: https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPGProviderSummary022817.pdf Urine Drug Testing (UDT) Federal Work Place Cut Off Values3-9 Initial Drug Test Level Confirmatory Drug Test Detection Period After Confirmatory -
![Chapter 329 [New] Uniform Controlled Substances Act](https://docslib.b-cdn.net/cover/1442/chapter-329-new-uniform-controlled-substances-act-1221442.webp)
Chapter 329 [New] Uniform Controlled Substances Act
CHAPTER 329 [NEW] UNIFORM CONTROLLED SUBSTANCES ACT Part I. General Provisions Section 329-1 Definitions 329-2 Hawaii advisory commission on drug abuse and controlled substances; number; appointment 329-3 Annual report 329-4 Duties of the commission Part II. Standards and Schedules 329-11 Authority to schedule controlled substances 329-12 Nomenclature 329-13 Schedule I tests 329-14 Schedule I 329-15 Schedule II tests 329-16 Schedule II 329-17 Schedule III Tests 329-18 Schedule III 329-19 Schedule IV tests 329-20 Schedule IV 329-21 Schedule V tests 329-22 Schedule V 329-23 Republishing and distribution of schedules Part III. Regulation of Manufacture, Distribution, Prescription, and Dispensing of Controlled Substances 329-31 Rules 329-31.5 Clinics 329-32 Registration requirements 329-33 Registration 329-34 Revocation and suspension of registration 329-35 Order to show cause 329-36 Records of registrants 329-37 Filing requirements 329-38 Prescriptions 329-39 Labels 329-40 Methadone treatment programs Part IV. Offenses and Penalties 329-41 Prohibited acts B-penalties 329-42 Prohibited acts C-penalties 329-43 Penalties under other laws 329-43.5 Prohibited acts related to drug paraphernalia Amended 0612 1 329-44 Notice of conviction to be sent to licensing board, department of commerce and consumer affairs 329-45 Repealed 329-46 Prohibited acts related to visits to more than one practitioner to obtain controlled substance prescriptions 329-49 Administrative penalties 329-50 Injunctive relief Part V. Enforcement and Administrative Provisions 329-51 Powers of enforcement personnel 329-52 Administrative inspections 329-53 Injunctions 329-54 Cooperative arrangements and confidentiality 329-55 Forfeitures 329-56 Burden of proof; liabilities 329-57 Judicial review 329-58 Education and research 329-59 Controlled substance registration revolving fund; established Part VI. -

Appendix-2Final.Pdf 663.7 KB
North West ‘Through the Gate Substance Misuse Services’ Drug Testing Project Appendix 2 – Analytical methodologies Overview Urine samples were analysed using three methodologies. The first methodology (General Screen) was designed to cover a wide range of analytes (drugs) and was used for all analytes other than the synthetic cannabinoid receptor agonists (SCRAs). The analyte coverage included a broad range of commonly prescribed drugs including over the counter medications, commonly misused drugs and metabolites of many of the compounds too. This approach provided a very powerful drug screening tool to investigate drug use/misuse before and whilst in prison. The second methodology (SCRA Screen) was specifically designed for SCRAs and targets only those compounds. This was a very sensitive methodology with a method capability of sub 100pg/ml for over 600 SCRAs and their metabolites. Both methodologies utilised full scan high resolution accurate mass LCMS technologies that allowed a non-targeted approach to data acquisition and the ability to retrospectively review data. The non-targeted approach to data acquisition effectively means that the analyte coverage of the data acquisition was unlimited. The only limiting factors were related to the chemical nature of the analyte being looked for. The analyte must extract in the sample preparation process; it must chromatograph and it must ionise under the conditions used by the mass spectrometer interface. The final limiting factor was presence in the data processing database. The subsequent study of negative MDT samples across the North West and London and the South East used a GCMS methodology for anabolic steroids in addition to the General and SCRA screens.