Bioactive Compounds in Waste By-Products from Olive Oil Production: Applications and Structural Characterization by Mass Spectrometry Techniques
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
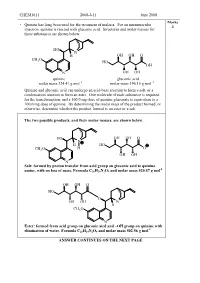
Oh Ho Oh Oh Oh Oh O Ho Ch3o N N H O Ho Oh Oh Oh Oh O Ho Ch3o N N
CHEM1611 2008-J-11 June 2008 Marks • Quinine has long been used for the treatment of malaria. For an intramuscular 4 injection, quinine is reacted with gluconic acid. Structures and molar masses for these substances are shown below. HO N H OH OH O CH O 3 HO OH N OH OH quinine gluconic acid molar mass 324.41 g mol –1 molar mass 196.16 g mol –1 Quinine and gluconic acid can undergo an acid-base reaction to form a salt, or a condensation reaction to form an ester. One molecule of each substance is required for the transformation, and a 160.0 mg dose of quinine gluconate is equivalent to a 100.0 mg dose of quinine. By determining the molar mass of the product formed, or otherwise, determine whether the product formed is an ester or a salt. The two possible products, and their molar masses, are shown below. HO OH OH O N H HO CH3O H O OH OH N Salt: formed by proton transfer from acid group on gluconic acid to quinine -1 amine, with no loss of mass. Formula C 26 H26 N2O9 and molar mass 520.57 g mol OH OH O HO O OH OH N H CH3O N Ester: formed from acid group on gluconic aicd and –OH group on quinine with -1 elimination of water. Formula C 26 H24 N2O8 and molar mass 502.56 g mol ANSWER CONTINUES ON THE NEXT PAGE CHEM1611 2008-J-11 June 2008 100.0 mg of quinine corresponds to: ̡̭̳̳ ̊̉̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.083 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̌̋̍.̍̊ ̧ ̭̯̬ ̊ 160.0 mg of the salt product corresponds to: ̡̭̳̳ ̊̏̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.074 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̎̋̉.̎̐ ̧ ̭̯̬ ̊ 160.0 mg of the ester product corresponds to: ̡̭̳̳ ̊̏̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.184 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̎̉̋.̎̏ ̧ ̭̯̬ ̊ As the dosages are the same, it must be the salt which is being administered. -

Assessment of Maternal Effects and Genetic Variability in Resistance to Verticillium Dahliae in Olive Progenies
plants Article Assessment of Maternal Effects and Genetic Variability in Resistance to Verticillium dahliae in Olive Progenies Pedro Valverde Caballero , Carlos Trapero Ramírez , Diego Barranco Navero, Francisco J. López-Escudero, Ana Gordon Bermúdez-Coronel and Concepción Muñoz Díez * Excellence Unit ‘María de Maeztu’ 2020-23, Department of Agronomy, ETSIAM, University of Córdoba, 14071 Córdoba, Spain; [email protected] (P.V.C.); [email protected] (C.T.R.); [email protected] (D.B.N.); [email protected] (F.J.L.-E.); [email protected] (A.G.B.-C.) * Correspondence: [email protected] Abstract: The use of genetic resistance is likely the most efficient, economically convenient and environmentally friendly control method for plant diseases, as well as a fundamental piece in an integrated management strategy. This is particularly important for woody crops affected by diseases in which mainly horizontal resistance mechanisms are operative, such as Verticillium wilt, caused by Verticillium dahliae. In this study, we analyzed the variability in resistance to Verticillium wilt of olive trees in progenies from five crosses: ‘Picual’ × ‘Frantoio’, ‘Arbosana’ × ‘Koroneiki’, ‘Sikitita’ × Citation: Valverde Caballero, P.; ‘Arbosana’, ‘Arbosana’ × ‘Frantoio’ and ‘Arbosana’ × ‘Arbequina’ and their respective reciprocal Trapero Ramírez, C.; Barranco crosses. Additionally, seedlings of ‘Picual’ and ‘Frantoio’ in open pollination were used as controls. Navero, D.; López-Escudero, F.J.; In October 2016 and 2018, the fruits were harvested, and seeds germinated. Six-week-old seedlings Gordon Bermúdez-Coronel, A.; Díez, were inoculated by dipping their bare roots in a conidial suspension of V. dahliae, and disease progress C.M. Assessment of Maternal Effects in terms of symptom severity and mortality was evaluated weekly. -

Agricultura Revista Agropecuaria, ISSN: 0002-1334
AGRICULTURA 2 copia:Maquetación 1 4/5/10 11:10 Página 362 DOSSIER PROGRAMA DE MEJORA ‘Sikitita’, nueva variedad para plantaciones de olivar en seto Foto 1. Vigor y hábito de crecimiento de ‘Sikitita’ (izquierda), ‘Arbequina’ (centro) y ‘Frantoio’ (derecha) a los siete años desde la plantación Luís Rallo ‘Sikitita’ es una nueva variedad de olivo procedente de un cruzamiento Diego Barranco Departamento de entre ‘Picual’ y ‘Arbequina’. Los resultados de la evaluación agronómica Agronomía, Universidad llevada a cabo en Córdoba han permitido su selección y registro como de Córdoba. una nueva variedad de precoz entrada en producción, alto contenido en Raúl de la Rosa aceite y elevada productividad. Su reducido vigor, su porte llorón y su Lorenzo León IFAPA Centro Alameda del alta densidad de ramos proporcionan una variedad particularmente Obispo. Córdoba. adaptada a las nuevas plantaciones de muy alta densidad en seto. Sikitita’ es la primera variedad ORIGEN Córdoba. La primera cosecha con los tres genitores del progra- desarrollada en el programa se obtuvo en 1996 y la evalua- ma original (‘Arbequina’, ‘Fran- ‘de mejora conjunto que se lle- La planta originaria de ‘Sikitita’ ción inicial de la planta se efec- toio’ y ‘Picual’) como testigos va a cabo entre la Universidad de (código UC-I 8-7 del programa de tuó durante tres cosechas con- fueron propagados por estaqui- Córdoba y el IFAPA. mejora) procede de un cruza- secutivas. La selección de la llado semileñoso en la primave- Este artículo resume la infor- miento entre ‘Picual’ (parental planta original de ‘Sikitita’ fue de- ra del año 2000 y se plantaron en mación obtenida hasta la fecha femenino) y ‘Arbequina’ (pa- bida a su precocidad de entrada un ensayo comparativo en blo- de esta nueva variedad e infor- rental masculino) llevado a cabo en producción (período juvenil ques al azar con 16 repeticiones ma sobre el estado actual de su en 1991 (Rallo, 1995). -

Promising Strain of Acinetobacter from Soil for Utilization of Gluconic Acid Production Topraktaki Gelecek Vaadeden Acinetobacte
S. Dinç et al. / Hacettepe J. Biol. & Chem., 2017, 45 (4), 603-607 Promising Strain of Acinetobacter from Soil for Utilization of Gluconic Acid Production Topraktaki Gelecek Vaadeden Acinetobacter Suşunun Glukonik Asit Üretiminde Kullanımı Research Article Saliha Dinç 1,2*, Meryem Kara2,3, Mehmet Öğüt3, Fatih Er1, Hacer Çiçekçi2 1Selcuk University Cumra School of Applied Sciences, Konya-Turkey. 2Selcuk Uni. Advanced Technology Research and Application Center, Konya-Turkey. 3Selcuk University Cumra Vocational High School, Konya-Turkey. ABSTRACT luconic acid, a food additive, is used in many foods to control acidity or binds metals such as calcium, Giron. Acinetobacter sp. WR326, newly isolated from soil possesses high phosphate solubilizing activity and do not require pyrroloquinoline quinone (PQQ) for glucose dehydrogenase (GDH) activity as cofactor In this study the gluconic acid production potential of this bacterium was investigated. Firstly, Acinetobacter sp. WR326 was incubated in tricalcium phosphate medium (TCP) with varying glucose concentrations (100, 250, 500 mM), at a temperature of 30°C for 5 days (120 hours). The highest gluconic acid yield (59%) was found at a glucose concentration of 100 mM. Then three different levels of gluconic acid addition to the medium (50, 100, 200 mM) were tested. When Acinetobacter sp. WR326 strain was cultivated with a 100 mM glucose and 100 mM gluconic acid the yield increased to 95.27%. In any trials 2 -keto D-gluconic acid, causes problems in processing and purification of the gluconic acid, was not detected in the medium. As a conclusion, Acinetobac- ter sp. WR326 may be considered novel potential bacterial strain for gluconic acid production. -

Assessment of Maternal Effects and Genetic Variability in Resistance to Verticillium Dahliae in Olive Progenies
Article Assessment of Maternal Effects and Genetic Variability in Resistance to Verticillium dahliae in Olive Progenies Pedro Valverde Caballero, Carlos Trapero Ramírez, Diego Barranco Navero, Francisco J. López-Escudero, Ana Gordon Bermúdez-Coronel and Concepción Muñoz Díez * Department of Agronomy (Excellence Unit ‘María de Maeztu’ 2020-23), ETSIAM, University of Córdoba, 14071 Córdoba, Spain; [email protected] (P.V.C.); [email protected] (C.T.R.); [email protected] (D.B.N.); [email protected] (F.J.L.-E.); [email protected] (A.G.B.-C.) * Correspondence: [email protected] Abstract: The use of genetic resistance is likely the most efficient, economically convenient and en- vironmentally friendly control method for plant diseases, as well as a fundamental piece in an inte- grated management strategy. This is particularly important for woody crops affected by diseases in which mainly horizontal resistance mechanisms are operative, such as Verticillium wilt, caused by Verticillium dahliae. In this study, we analyzed the variability in resistance to Verticillium wilt of olive trees in progenies from five crosses: ‘Picual’ × ‘Frantoio’, ‘Arbosana’ × ‘Koroneiki’, ‘Sikitita’ × Citation: Valverde, P.; Trapero, C.; ‘Arbosana’, ‘Arbosana’ × ‘Frantoio’ and ‘Arbosana’ × ‘Arbequina’ and their respective reciprocal Barranco, D.; López-Escudero, F.J.; crosses. Additionally, seedlings of ‘Picual’ and ‘Frantoio’ in open pollination were used as controls. Gordon, A.; Díez C. M. Assessment In October 2016 and 2018, the fruits were harvested, and seeds germinated. Six-week-old seedlings of maternal effect and genetic varia- were inoculated by dipping their bare roots in a conidial suspension of V. dahliae, and disease pro- bility in resistance to Verticillium gress in terms of symptom severity and mortality was evaluated weekly. -

Biosynthesis of Abscisic Acid by the Direct Pathway Via Ionylideneethane in a Fungus, Cercospora Cruenta
Biosci. Biotechnol. Biochem., 68 (12), 2571–2580, 2004 Biosynthesis of Abscisic Acid by the Direct Pathway via Ionylideneethane in a Fungus, Cercospora cruenta y Masahiro INOMATA,1 Nobuhiro HIRAI,2; Ryuji YOSHIDA,3 and Hajime OHIGASHI1 1Division of Food Science and Biotechnology, Graduate School of Agriculture, Kyoto University, Kyoto 606-8502, Japan 2International Innovation Center, Kyoto University, Kyoto 606-8501, Japan 3Department of Agriculture Technology, Toyama Prefectural University, Toyama 939-0311, Japan Received August 11, 2004; Accepted September 12, 2004 We examined the biosynthetic pathway of abscisic Key words: Cercospora cruenta; abscisic acid; allofar- acid (ABA) after isopentenyl diphosphate in a fungus, nesene; -ionylideneethane; all-E-7,8-dihy- Cercospora cruenta. All oxygen atoms at C-1, -1, -10, and dro- -carotene -40 of ABA produced by this fungus were labeled with 18 18 O from O2. The fungus did not produce the 9Z- A sesquiterpenoid, abscisic acid (ABA, 1), is a plant carotenoid possessing -ring that is likely a precursor hormone which regulates seed dormancy and induces for the carotenoid pathway, but produced new sesqui- dehydration tolerance by reducing the stomatal aper- terpenoids, 2E,4E- -ionylideneethane and 2Z,4E- -ion- ture.1) ABA is biosynthesized by some phytopathogenic ylideneethane, along with 2E,4E,6E-allofarnesene. The fungi in addition to plants,2) but the biosynthetic origin fungus converted these sesquiterpenoids labeled with of isopentenyl diphosphate (IDP) for fungal ABA is 13C to ABA, and the incorporation ratio of 2Z,4E- - different from that for plant ABA (Fig. 1). Fungi use ionylideneethane was higher than that of 2E,4E- - IDP derived from the mevalonate pathway for ABA, ionylideneethane. -

Bioactive Compounds of Tomatoes As Health Promoters
48 Natural Bioactive Compounds from Fruits and Vegetables, 2016, Ed. 2, 48-91 CHAPTER 3 Bioactive Compounds of Tomatoes as Health Promoters José Pinela1,2, M. Beatriz P. P Oliveira2, Isabel C.F.R. Ferreira1,* 1 Mountain Research Centre (CIMO), ESA, Polytechnic Institute of Bragança, Campus de Santa Apolónia, Ap. 1172, 5301-855 Bragança, Portugal 2 REQUIMTE/LAQV, Faculty of Pharmacy, University of Porto, Rua Jorge Viterbo Ferreira, n° 228, 4050-313 Porto, Portugal Abstract: Tomato (Lycopersicon esculentum Mill.) is one of the most consumed vegetables in the world and probably the most preferred garden crop. It is a key component of the Mediterranean diet, commonly associated with a reduced risk of chronic degenerative diseases. Currently there are a large number of tomato cultivars with different morphological and sensorial characteristics and tomato-based products, being major sources of nourishment for the world’s population. Its consumption brings health benefits, linked with its high levels of bioactive ingredients. The main compounds are carotenoids such as β-carotene, a precursor of vitamin A, and mostly lycopene, which is responsible for the red colour, vitamins in particular ascorbic acid and tocopherols, phenolic compounds including hydroxycinnamic acid derivatives and flavonoids, and lectins. The content of these compounds is variety dependent. Besides, unlike unripe tomatoes, which contain a high content of tomatine (glycoalkaloid) but no lycopene, ripe red tomatoes contain high amounts of lycopene and a lower quantity of glycoalkaloids. Current studies demonstrate the several benefits of these bioactive compounds, either isolated or in combined extracts, namely anticarcinogenic, cardioprotective and hepatoprotective effects among other health benefits, mainly due to its antioxidant and anti-inflammatory properties. -

LA PRODUCTIVITE D'un VERGER D'olivier Eléments De Réflexion
LA PRODUCTIVITE D’UN VERGER D’OLIVIER Eléments de réflexion Gordes, 30 septembre 2020 Hélène LASSERRE France Olive/ Pôle Conservation Recherche LA FILIÈRE OLÉICOLE FRANÇAISE 2020 Présentation de l’interprofession FRANCE OLIVE C’EST : ➢ L’association française interprofessionnelle de l’olive (ex. AFIDOL) ➢ Une association, reconnue par l’État, créée en 1999 ➢ Une représentation de tous les acteurs de la filière oléicole ➢ Un accord interprofessionnel triennal signé par les familles représentatives ➢ Un budget de 2 000 k€ financé pour : ⚫ 40 % : par les Cotisations Volontaires Etendues* de l’amont et de l’aval ⚫ 60 % : par les subventions européennes, nationales et régionales ➢ Trois antennes dans les trois principales régions productrices : Région Occitanie Région Sud, Provence-Alpes-Côte d’Azur Région Auvergne-Rhône-Alpes Mas de l'agriculture Maison des Agriculteurs 40, place de la Libération 1120, route de Saint-Gilles 22, avenue Henri Pontier 26110 Nyons 30932 Nîmes 13626 Aix-en-Provence Tél. 04 75 26 90 90 Tél. 04 66 08 19 34 Tél. 04 42 23 01 92 * CVE (ex. CVO) : « Volontaires » car décidées par les familles représentatives de la filière et « Etendues » car rendues obligatoires par l’Etat à l’ensemble des acteurs de la filière par extension de l’accord interprofessionnel. 3 millions de tonnes 5 000 tonnes 0,15% de la production mondiale 2,1 millions de tonnes Une production emblématique pour les régions du Sud de la France mais anecdotique au niveau mondial. Consommation française totale d'huile d'olive 108 000 tonnes Production française 5 000 tonnes Part dans la consommation nationale : 4 % - Une production issue d'entreprises familiales et artisanales - 25 % de la production sous label AOP (8 appellations d’origine) issue de variétés locales, typiques et uniques et une production « bio » importante (28% du verger) - Trois familles de goûts et une multitude de variantes au sein de chaque famille : en fonction de la ou des variétés, du terroir et du savoir-faire, les goûts de l’huile d’olive sont différents. -

Μedals & Special Prizes
5th Αthena International Olive Oil Competition • SPATA • June 11–13 2020 ΜEDALS & SPECIAL PRIZES Final Participation and Awards Results DOUBLE GOLD DOUBLE GOLD MEDALS EVOOIL EVOO PRODUCER VARIETAL MAKE UP COUNTRY REGION WEBSITE FLAVOR ΒΙΟ Longnan Xiangyu Olive Xiangyu Coratina Coratina China Longnan, Gansu www.xiangyuoliveoil.com Development ✓ Mitera Raio Mitera Rajo Italy Umbria, Perugia www.mitera.ch Kyklopas Early Harvest Kyklopas Makris Greece Thrace, Evros www.kyklopas.com Aprutino Pescarese Sandro di Azienda Agricola Sandro di 80% Dritta, Italy Abruzzo, Pescara Giacomo Giacomo 20% Intosso Picualia Premium Reserva Picualia Picual Spain Andalusia, Jaén www.picualia.com Il Re dei Sassi Le Mandrie Moraiolo Italy Umbria, Perugia www.agriturismomandriesanpaolo.it ✓ Jeff’s Blend Fedra Olive Grove Frantoio Australia New South Wales, Collector www.fedraolivegrove.com.au 70% Koroneiki, Cretanthos Early Harvest Organic Cretanthos Greece Crete, Rethymno www.cretanthos.gr 30% Tsounati ✓ Bose Oil Bose Oil Briška Črnica Slovenia Goriška, Goriška Brda Iliada Agrovim Koroneiki Greece Peloponnese, Messenia www.agrovim.gr Domaine Petraghje Domaine Petraghje Germana di Casinca France Corsica, Haute-Corse Organic Biodynamic Picudo Cortijo el Puerto Picudo Spain Andalusia, Sevilla www.cortijoelpuerto.com ✓ Organic Biodynamic Hojiblanca Cortijo el Puerto Hojiblanca Spain Andalusia, Sevilla www.cortijoelpuerto.com ✓ Mediterre Olympia Organic Early 90% Koroneiki, Mediterre Eurofood Greece Peloponnese, Elis www.mediterre.com Harvest 10% Kolireiki ✓ 45% Hojiblanca, -

Figure S1. Heat Map of R (Pearson's Correlation Coefficient)
Figure S1. Heat map of r (Pearson’s correlation coefficient) value among different samples including replicates. The color represented the r value. Figure S2. Distributions of accumulation profiles of lipids, nucleotides, and vitamins detected by widely-targeted UPLC-MC during four fruit developmental stages. The colors indicate the proportional content of each identified metabolites as determined by the average peak response area with R scale normalization. PS1, 2, 3, and 4 represents fruit samples collected at 27, 84, 125, 165 Days After Anthesis (DAA), respectively. Three independent replicates were performed for each stages. Figure S3. Differential metabolites of PS2 vs PS1 group in flavonoid biosynthesis pathway. Figure S4. Differential metabolites of PS2 vs PS1 group in phenylpropanoid biosynthesis pathway. Figure S5. Differential metabolites of PS3 vs PS2 group in flavonoid biosynthesis pathway. Figure S6. Differential metabolites of PS3 vs PS2 group in phenylpropanoid biosynthesis pathway. Figure S7. Differential metabolites of PS4 vs PS3 group in biosynthesis of phenylpropanoids pathway. Figure S8. Differential metabolites of PS2 vs PS1 group in flavonoid biosynthesis pathway and phenylpropanoid biosynthesis pathway combined with RNA-seq results. Table S1. A total of 462 detected metabolites in this study and their peak response areas along the developmental stages of apple fruit. mix0 mix0 mix0 Index Compounds Class PS1a PS1b PS1c PS2a PS2b PS2c PS3a PS3b PS3c PS4a PS4b PS4c ID 1 2 3 Alcohols and 5.25E 7.57E 5.27E 4.24E 5.20E -

The Possibility of Recovering of Hydroxytyrosol from Olive Milling Wastewater by Enzymatic Bioconversion 265
ProvisionalChapter chapter 14 The PossibilityPossibility of of Recovering Recovering of Hydroxytyrosolof Hydroxytyrosol from fromOlive Milling Wastewater by Enzymatic Bioconversion Olive Milling Wastewater by Enzymatic Bioconversion Manel Hamza and Sami Sayadi Manel Hamza and Sami Sayadi Additional information is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/64774 Abstract This chapter discusses an innovative approach to obtain liquid fractions from olive mill wastewater (OMW) rich in hydroxytyrosol. The method is based on bioconversion combined with membrane separation techniques. An enzymatic bioconversion of three types of OMW was tested. TheS total volumes of OMW are 15 and 40 L. The reaction was monitored in mechanically stirred systems for 2 h at 50°C. Maximum hydroxytyr‐ osol concentrations of about 1.53, 0.83 and 0.46 g/L in the presence of 5 IU Aspergillus niger β‐glucosidase per milliliter from North OMW and South OMW were procured by two different olive millings, which are milling super press (MSP) and milling continuous chain (MCC), respectively. Enzymatic pretreatment was followed by two tangential flow membrane separation stages, microfiltration (MF) and ultrafiltration (UF). The ultrafiltration permeate was concentrated by evaporation at 45°C for 2 h. The latter exhibited a chemical oxygen demand (COD) level of 48.44 g/L. The UF permeate dehydration increased the hydroxytyrosol concentration to 7.2 g/L. A new natural product that contains some minerals beneficial to health and devoid of heavy metals or chemicals was obtained by this innovative work which describes an environmentally friendly process at pilot‐scale. -
Multiplicity of Carotene Patterns Derives from Competition Between
www.nature.com/scientificreports OPEN Multiplicity of carotene patterns derives from competition between phytoene desaturase diversifcation and biological environments Mathieu Fournié1,2,3 & Gilles Truan1* Phytoene desaturases catalyse from two to six desaturation reactions on phytoene, generating a large diversity of molecules that can then be cyclised and produce, depending on the organism, many diferent carotenoids. We constructed a phylogenetic tree of a subset of phytoene desaturases from the CrtI family for which functional data was available. We expressed in a bacterial system eight codon optimized CrtI enzymes from diferent clades. Analysis of the phytoene desaturation reactions on crude extracts showed that three CrtI enzymes can catalyse up to six desaturations, forming tetradehydrolycopene. Kinetic data generated using a subset of fve purifed enzymes demonstrate the existence of characteristic patterns of desaturated molecules associated with various CrtI clades. The kinetic data was also analysed using a classical Michaelis–Menten kinetic model, showing that variations in the reaction rates and binding constants could explain the various carotene patterns observed. Competition between lycopene cyclase and the phytoene desaturases modifed the distribution between carotene intermediates when expressed in yeast in the context of the full β-carotene production pathway. Our results demonstrate that the desaturation patterns of carotene molecules in various biological environments cannot be fully inferred from phytoene desaturases classifcation but is governed both by evolutionary-linked variations in the desaturation rates and competition between desaturation and cyclisation steps. Carotenoids are organic pigments produced by plants, algae, fungi, and bacteria and can be subdivided into two families of molecules, carotenes and their oxidised counterparts, xanthophylls 1.