Allogeneic Anorectal Transplantation in Rats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Anatomy of Th-E Blood Vascular System of the Fox ,Squirrel
THE ANATOMY OF TH-E BLOOD VASCULAR SYSTEM OF THE FOX ,SQUIRREL. §CIURUS NlGER. .RUFIVENTEB (OEOEEROY) Thai: for the 009m of M. S. MICHIGAN STATE COLLEGE Thomas William Jenkins 1950 THulS' ifliillifllfllilllljllljIi\Ill\ljilllHliLlilHlLHl This is to certifg that the thesis entitled The Anatomy of the Blood Vascular System of the Fox Squirrel. Sciurus niger rufiventer (Geoffroy) presented by Thomas William Jenkins has been accepted towards fulfillment of the requirements for A degree in MEL Major professor Date May 23’ 19500 0-169 q/m Np” THE ANATOMY OF THE BLOOD VASCULAR SYSTEM OF THE FOX SQUIRREL, SCIURUS NIGER RUFIVENTER (GEOFFROY) By THOMAS WILLIAM JENKINS w L-Ooffi A THESIS Submitted to the School of Graduate Studies of Michigan State College of Agriculture and Applied Science in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Department of Zoology 1950 \ THESlSfi ACKNOWLEDGMENTS Grateful acknowledgment is made to the following persons of the Zoology Department: Dr. R. A. Fennell, under whose guidence this study was completed; Mr. P. A. Caraway, for his invaluable assistance in photography; Dr. D. W. Hayne and Mr. Poff, for their assistance in trapping; Dr. K. A. Stiles and Dr. R. H. Manville, for their helpful suggestions on various occasions; Mrs. Bernadette Henderson (Miss Mac), for her pleasant words of encouragement and advice; Dr. H. R. Hunt, head of the Zoology Department, for approval of the research problem; and Mr. N. J. Mizeres, for critically reading the manuscript. Special thanks is given to my wife for her assistance with the drawings and constant encouragement throughout the many months of work. -

A Rare Variation of the Inferior Mesenteric Vein with Clinical
CASE REPORT A rare variation of the inferior mesenteric vein with clinical implications Danielle Park, Sarah Blizard, Natalie O’Toole, Sheeva Norooz, Martin Dela Torre, Young Son, Michael McGuinness, Mei Xu Park D, Blizard S, O’Toole N, et al. A rare variation of the inferior the middle colic vein. The superior mesenteric vein then united with the mesenteric vein with clinical implications. Int J Anat Var. Mar 2019;12(1): splenic vein to become the hepatic portal vein. Awareness of this uncommon 024-025. anatomy of the inferior mesenteric vein is important in planning a successful gastrointestinal surgery. Several variations of the inferior mesenteric vein have been previously described. However, this report presents a rare variation that has not yet been noted. In this case, the small inferior mesenteric vein drained into a Key Words: Inferior mesenteric vein; Marginal vein; Middle colic vein; Superior tributary of the marginal vein, which joined the superior mesenteric vein via mesenteric vein INTRODUCTION he portal venous system consists of four large veins: the hepatic portal, Tsplenic (SV), superior mesenteric (SMV) and inferior mesenteric (IMV). The SMV collects the venous return from the small intestine, stomach, pancreas, cecum, ascending colon and proximal portion of the transverse colon. The SMV tributaries include the small intestine, right gastro-omental, inferior pancreaticoduodenal, ileocolic, right colic, middle colic (MCV) and marginal (MarV) veins. The IMV receives the blood from the superior rectal, sigmoid and left colic veins, which cover the distal portion of the transverse colon, descending colon, sigmoid colon and superior rectum. According to the description by Thompson in 1890, the portal vein tributaries are categorized into four types [1]. -
A Rare Presentation of Duplicated Inferior Vena Cava in a Donor
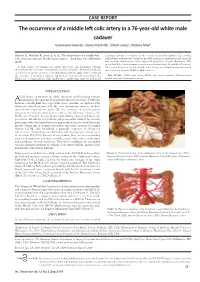
CASE REPORT The occurrence of a middle left colic artery in a 76-year-old white male cadaver Guinevere Granite1, Keiko Meshida2, Shiloh Jones3, Natalie May4 Granite G, Meshida K, Jones S, et al. The occurrence of a middle left teaching anatomy to students in the various medical disciplines. Case studies colic artery in a 76-year-old white male cadaver . Int J Anat Var. 2019;12(3): highlighting such vascular variations provide anatomical instructors and surgeons 26-29. with accurate information on the types and prevalence of such alterations. This article highlights an abdominal vascular variation involving the middle colic artery. A high degree of variation in origin, trajectory, and branching patterns This vessel, known as the left middle colic artery, was found during anatomical characterizes the anatomy of mesenteric vascular structures. Detailed knowledge dissection of a 76-year-old White Male cadaver. of normal and variant anatomy of the abdominal arterial supply serves to improve the outcome of oncologic, surgical, radiological interventions and reduces the Key Words: Middle colic artery; Middle colic artery variation; Gastrointestinal likelihood of complications. Such familiarity is equally important for instructors arterial variation; Anatomical variation INTRODUCTION high degree of variation in origin, trajectory, and branching patterns Acharacterizes the anatomy of mesenteric vascular structures. Textbooks, however, critically limit the scope of the classic anatomic description of the abdominal arterial pattern [1,2]. Yet, such descriptions serve as the basis upon which many surgeons operate [1]. The occurrence of vascular pattern variations in common surgical sites, such as the abdomen, increases the likelihood of vascular damage by specialists during surgical and diagnostic procedures. -

Colon Operative Standards
282 SECTION IV | COLON F G E F FIGURE 16-7 (Continued). patients with hereditary nonpolyposis colon cancer, as they have a higher incidence of synchronous and metachronous colonic tumors than do patients with sporadic colorectal cancer. As calculated by life table analysis, the risk for metachronous cancer among patients with hereditary nonpolyposis is as high as 40% at 10 years. Simi- larly, for colon cancer patients with familial adenomatous polyposis, surgical resec- tion should consist of either total abdominal colectomy or total proctocolectomy. The choice between these two operations depends on the burden of polypoid disease in the rectum and the patient’s preference for close surveillance. 7,8,9 Finally, individuals who develop colon cancer in the setting of long-standing ulcerative colitis require a total proctocolectomy. The oncologic principles of colon cancer surgery as outlined in this chapter, including the attention to surgical margins and the need for proximal vascular ligation, should be adhered to bilaterally, not just for the portion of colon in which the tumor has been identifi ed.10,11 3. PROXIMAL VASCULAR LIGATION AND REGIONAL LYMPHADENECTOMY Recommendation: Resection of the tumor-bearing bowel segment and radical lymphadenectomy should be performed en bloc with proximal vascular ligation at the origin of the primary feeding vessel(s). Copyright © 2015 Wolters Kluwer Health, Inc. Unauthorized reproduction of the article is prohibited. 226_ACS_Ch16.indd6_ACS_Ch16.indd 228282 44/3/15/3/15 22:58:58 AAMM CHAPTER 16 | Colon Resection 283 Type of Data: Prospective and retrospective observational studies. Strength of Recommendation: Moderate. Rationale The standard of practice for the treatment of stage I to III (nonmetastatic) colon can- cer is complete margin-negative resection (R0 resection) of the tumor-bearing bowel combined with en bloc resection of the intact node-bearing mesentery (i.e., regional lymphadenectomy). -

SŁOWNIK ANATOMICZNY (ANGIELSKO–Łacinsłownik Anatomiczny (Angielsko-Łacińsko-Polski)´ SKO–POLSKI)
ANATOMY WORDS (ENGLISH–LATIN–POLISH) SŁOWNIK ANATOMICZNY (ANGIELSKO–ŁACINSłownik anatomiczny (angielsko-łacińsko-polski)´ SKO–POLSKI) English – Je˛zyk angielski Latin – Łacina Polish – Je˛zyk polski Arteries – Te˛tnice accessory obturator artery arteria obturatoria accessoria tętnica zasłonowa dodatkowa acetabular branch ramus acetabularis gałąź panewkowa anterior basal segmental artery arteria segmentalis basalis anterior pulmonis tętnica segmentowa podstawna przednia (dextri et sinistri) płuca (prawego i lewego) anterior cecal artery arteria caecalis anterior tętnica kątnicza przednia anterior cerebral artery arteria cerebri anterior tętnica przednia mózgu anterior choroidal artery arteria choroidea anterior tętnica naczyniówkowa przednia anterior ciliary arteries arteriae ciliares anteriores tętnice rzęskowe przednie anterior circumflex humeral artery arteria circumflexa humeri anterior tętnica okalająca ramię przednia anterior communicating artery arteria communicans anterior tętnica łącząca przednia anterior conjunctival artery arteria conjunctivalis anterior tętnica spojówkowa przednia anterior ethmoidal artery arteria ethmoidalis anterior tętnica sitowa przednia anterior inferior cerebellar artery arteria anterior inferior cerebelli tętnica dolna przednia móżdżku anterior interosseous artery arteria interossea anterior tętnica międzykostna przednia anterior labial branches of deep external rami labiales anteriores arteriae pudendae gałęzie wargowe przednie tętnicy sromowej pudendal artery externae profundae zewnętrznej głębokiej -

Netter's Anatomy Flash Cards – Section 4 – List 4Th Edition
Netter's Anatomy Flash Cards – Section 4 – List 4th Edition https://www.memrise.com/course/1577335/ Section 4 Abdomen (31 cards) Plate 4-1 Bony Framework of Abdomen 1.1 Costal cartilages 1.2 Iliac crest 1.3 Anterior superior iliac spine 1.4 Anterior inferior iliac spine 1.5 Superior pubic ramus 1.6 Pubic arch 1.7 Pecten pubis 1.8 Greater trochanter of femur 1.9 Ischial spine 1.10 Iliac crest 1.11 Xiphoid process 1.12 Body of sternum Plate 4-2 Anterior Abdominal Wall: Superficial Dissection 2.1 External oblique muscle: muscular part (A) and aponeurotic part (B) Plate 4-3 Anterior Abdominal Wall 3.1 Internal oblique muscle Plate 4-4 Anterior Abdominal Wall 4.1 Rectus abdominis muscle Plate 4-5 Anterior Abdominal Wall 5.1 Cremaster muscle Plate 4-6 Anterior Abdominal Wall: 6.1 Superior epigastric vessels 6.2 Rectus abdominis muscle 6.3 Transversus abdominis muscle 6.4 Posterior layer of rectus sheath 6.5 Inferior epigastric vessels 6.6 Inguinal ligament (Poupart’s ligament) 6.7 Inguinal falx (conjoint tendon) 6.8 Cremasteric muscle (middle spermatic fascia) 6.9 Lacunar ligament (Gimbernat’s ligament) 6.10 Medial umbilical ligament (occluded part of umbilical artery) 6.11 Arcuate line 6.12 Transversalis fascia 6.13 Anterior layer of rectus sheath 6.14 Linea alba Plate 4-7 Posterior Abdominal Wall: Internal View 7.1 Quadratus lumborum muscle Plate 4-8 Posterior Abdominal Wall: Internal View 8.1 Diaphragm Plate 4-9 Autonomic Nerves and Ganglia of Abdomen 9.1 Right greater and lesser splanchnic nerves 9.2 Right sympathetic trunk 9.3 2nd and -

Variations in Right Colic Vascular Anatomy Observed During
Wu et al. World Journal of Surgical Oncology (2019) 17:16 https://doi.org/10.1186/s12957-019-1561-4 RESEARCH Open Access Variations in right colic vascular anatomy observed during laparoscopic right colectomy Chuying Wu†, Kai Ye*†, Yiyang Wu, Qiwei Chen, Jianhua Xu, Jianan Lin and Wengui Kang Abstract Background: This study aimed to analyze right colonic vascular variability. Methods: The study included 60 consecutive patients who underwent laparoscopic radical right colectomy and D3 lymph node dissection for malignant colonic cancer on the ileocecal valve, ascending colon or hepatic flexure (March 2013 to October 2016). The videos of the 60 surgical procedures were collected. Variations of right colonic vascular anatomy were retrospectively analyzed based on 60 high-resolution surgical videos of laparoscopic surgery. Results: The superior mesenteric artery and vein were present in all cases; 95.0% (57/60) had the superior mesenteric artery on the left side of the superior mesenteric vein. The ileocolic artery and vein occurred in 96.7% (58/60) and 100% (60/60) of cases, respectively; 50.0% (29/58) had the ileocolic artery passing the superior mesenteric vein anteriorly. Thirty-three (55.0%) cases had a right colic artery, and 2 (3.33%) had a double right colic artery; 90.9% (30/36) had the right colic vein passing anterior to the superior mesenteric artery. Fifty-six (93.3%) cases had a right colic vein; 7 (12.5%) had a right colic vein accompanied by a right colic artery, 66.1% (37/56) had the right colic vein draining into the gastrocolic trunk of Henle, 23.2% (13/56) had the right colic vein directly draining into superior mesenteric vein, and 10.7% (6/56) had one right colic vein draining into the superior mesenteric vein and the other into the gastrocolic trunk of Henle. -

Hepatic Portal System (Advanced) USMLE, Limited Edition > Gross Anatomy > Gross Anatomy
Hepatic Portal System (Advanced) USMLE, Limited Edition > Gross Anatomy > Gross Anatomy Hepatic portal system • A special circulation system that transports venous blood from the digestive organs to the liver. • Transports blood from the stomach, spleen, pancreas, and small and large intestines to the liver. This distinct circulatory pathway exists to allow the liver to metabolize nutrients and toxins from blood that leaves the digestive organs. Primary tributaries of the hepatic portal vein: Superior mesenteric vein Drains tissues of the right side of the abdomen. - Ileocolic vein drains blood from the distal small intestine and the proximal large intestine - Right colic vein courses from the right side of the abdomen to drain blood from the large intestine - Middle colic vein drains blood from the large intestine. - Intestinal veins drain the jejunum and ileum of the small intestine. These drain into the left side of the superior mesenteric vein. - Pancreatic and duodenal veins - Right gastro-omental vein, which runs along the inferior border of the stomach (aka, greater curvature), drains into the superior mesenteric vein. • The "omental" portion of the gastro-omental name is derived from the greater "omentum," the apron-like fold of peritoneum that drapes over the intestines anteriorly. Splenic vein Drains structures on the left side of the abdomen. - Merges with superior mesenteric vein to form hepatic portal vein - Short gastric veins from stomach - Left gastro-omental vein, which courses along the inferior border of the stomach and meets the right gastro-omental vein. - Pancreatic veins - Inferior mesenteric vein Inferior mesenteric vein 1 / 2 Drains tissues of the lower left side of the abdomen into the splenic vein. -

Anatomy and Physiology Model Guide Book
Anatomy & Physiology Model Guide Book Last Updated: August 8, 2013 ii Table of Contents Tissues ........................................................................................................................................................... 7 The Bone (Somso QS 61) ........................................................................................................................... 7 Section of Skin (Somso KS 3 & KS4) .......................................................................................................... 8 Model of the Lymphatic System in the Human Body ............................................................................. 11 Bone Structure ........................................................................................................................................ 12 Skeletal System ........................................................................................................................................... 13 The Skull .................................................................................................................................................. 13 Artificial Exploded Human Skull (Somso QS 9)........................................................................................ 14 Skull ......................................................................................................................................................... 15 Auditory Ossicles .................................................................................................................................... -

Long-Term Oncologic Outcomes of Laparoscopic Surgery for Splenic Flexure Colon Cancer Are Comparable to Conventional Open Surgery
ORIGINAL ARTICLE pISSN 2288-6575 • eISSN 2288-6796 https://doi.org/10.4174/astr.2017.93.1.35 Annals of Surgical Treatment and Research Long-term oncologic outcomes of laparoscopic surgery for splenic flexure colon cancer are comparable to conventional open surgery Min Ki Kim, In Kyu Lee, Won-Kyung Kang1, Hyeon-Min Cho2, Bong-Hyeon Kye2, Heba Essam Jalloun, Jun-Gi Kim Department of Surgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, 1Department of Surgery, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, 2Department of Surgery, St. Vincent Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea Purpose: Few studies about laparoscopic surgery for splenic flexure colon cancer have been published. This study aims to compare the short- and long-term outcomes of laparoscopic surgery for splenic flexure colon cancer with those of con- ven tional open surgery. Methods: From January 2004 to December 2010, 51 consecutive patients who underwent curative resection for stages I– III splenic flexure colon cancer were enrolled. Thirty-three patients underwent laparoscopy-assisted colectomy, while 18 patients underwent conventional open colectomy. Short- and long-term outcomes of the 2 groups were compared. Results: There were no differences in baseline characteristics, intra- and postoperative complications. The laparoscopy group showed longer operation time (median [interquartile range, IQR]: 295.0 [255.0–362.5] minutes vs. 180.0 [168.8–206.3] minutes, P < 0.001). In the laparoscopy group, return of bowel function was faster (median [IQR]: 3 [2–4] vs. -

NUTRITIONAL ANALYSIS REPORT Name: Client#: Scan Tech Name: Company Name
NUTRITIONAL ANALYSIS REPORT Name: Client#: Scan Tech Name: Company Name: Dr Elian Duke (X Dr. Elian Duke 1 1 1 Email: Birthdate: Age: Scan Date: Q Address: > SCAN 8/7/2019 O 6000A Sawgrass Vil CLIENT rr' Gender: Height: Weight: Scan Time: Q_ Phone: 5:28:30 PM; 904 379-3443 Macrominerals (Continued) Chloride Magnesium Phosphorus Potassium Sodium Sulphur PreviousC 1 Scan Microminerals Low Normal High Low Normal High Boron Copper Chromium Fluorine n Germanium Iodine Iron Lithium Manganese Molybdenum 1 Selenium Silicon C J Zinc 1^^ Previous Scan Vitamins / CoEnzymes Low Normal High Low Normal High Bl, Thiamine B2, Riboflavin B3, Niacin B3, Nicotinamide B5, Pantothenic Acid B6, Pyridoxine 1 B7, Biotin C J B9, Folate B12, Cobalamin Beta Carotene CoEnzyme Q10 Vitamin A n Vitamin C Vitamin D, 25-Hydroxy Vitamin E Vitamin K Page 2 Frequency Optimization *Please see disclosure at end of report Name: Technician: Dr Elian Duke R Transverse Lat. Circumfl^ 5 R Medial Circumflex Artery 5 5 Module: Arterico-t R Lateral Circumflex 9[ffi 8/19/2019 1:11 R Deep Femoral Artery 5 5 R Descending Lat. Circumflgx 5 R Ascending Lat. Circumflefl 6 R Saphenous Descending Gemci^r R Femoral Artery 5 6 4 R Articular Descending Genicufir 5 R Popliteal Artery 4 4 R Medial Inferior Genicular 5 5 R Descending Genicular Arte’ y 7 R Lateral Inferior Genicufir 5 R Anterior Tibial Recurrefil 5 R Posterior Tibial Artery5 4 R Circumflex Fibular Arter£ 5 R Fibular Artery 5 5 R Medial Tarsal Arteries 4 5 R Lateral Tarsal Artery 6 6 R Dorsal Metatarsal Arteriefi 5 R Arcuate Artery -

Gastrocolic Trunk of Henle and Its Variants: Review of the Literature and Clinical Relevance in Colectomy for Right‑Sided Colon Cancer
Surgical and Radiologic Anatomy (2019) 41:879–887 https://doi.org/10.1007/s00276-019-02253-4 REVIEW Gastrocolic trunk of Henle and its variants: review of the literature and clinical relevance in colectomy for right‑sided colon cancer Roberto Peltrini1 · Gaetano Luglio1 · Gianluca Pagano1 · Michele Sacco1 · Viviana Sollazzo1 · Luigi Bucci1 Received: 11 March 2019 / Accepted: 4 May 2019 / Published online: 14 May 2019 © Springer-Verlag France SAS, part of Springer Nature 2019 Abstract Purpose Venous vascular anatomy of the right colon presents a high degree of variability. Henle’s Gastrocolic Trunk is considered an important anatomical landmark by colorectal surgeons. The classical description concerns a bipod vascular structure or tripod, but several variants are associated to it. The aim of this study is to merge the most updated literature on the anatomy knowledge of the Gastrocolic Trunk by evaluating all possible variants, as well as to underline its surgical importance due to its topographical relationships. Methods Twelve studies describing the anatomy of the gastrocolic trunk were selected, each of them dealing with a more or less extensive series of cases. A distinction was drawn between the gastropancreatic trunk, devoid of the colonic component, and the gastrocolic trunk; and then the frequency of the diferent resulting variants was reported. The data obtained from cadavers and radiological studies were analyzed separately. Results The Gastrocolic Trunk is found in 74% of cadaver studies, and in 86% of radiological studies. Its most frequent confguration is represented by the union of right gastroepiploic vein + anterior superior pancreaticoduodenal vein + superior right colic vein, respectively, 32.5% and 42.5%, followed by the right colic vein which replaces (26.9%, 12.3%) or is added (10%, 20.1%) to the superior right colic vein.