Targeting the PLK1-FOXO1 Pathway As a Novel Therapeutic Approach For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Abbreviation
Exploring and Potentiating Human Bone Marrow Derived Mesenchymal Stem Cells [hBMSCs] and Differentiated Islets for Effective Diabetes Therapy ABBREVIATION LIST OF ABBREVIATION AF555-Alexa Fluor 555 EBs- Embryoid bodies AGE- advanced glycosylation end-product EDTA- Ethylenediaminetetraacetic acid aPKC- Atypical protein kinase C EGF-Epidermal growth factor ANOVA-Analysis of variance EGFR- epidermal growth factor receptor Arx- Aristaless-related homeobox gene EL- Enicostemma littorale ATP- Adenosine triphosphate ELISA- Enzyme linked immunosorbent bHLH- Basic helix–loop–helix assay BM- Bone marrow EMT - epithelial–mesenchymal transition BMC- Bioactive molecules cocktail ESCs- Embryonic stem cells BMP-bone morphogenic protein FACS- Fluorescent activated cell sorter BSA- Bovine serum albumin FBS- Fetal Bovine Serum cAMP- Cyclic Adenosine monophosphate FDA- Food and Drug Administration CaCl2 –Calcium chloride FGF- Fibroblast growth factor CD-Cluster of differentiation FITC- Fluorescein isothiocyanate cDNA –Complementary DNA FOXO1-Forkhead box protein O1 ChIP-Chromatin immunoprecipitation FOXA2 - Forkhead box protein A2 CCl4- Carbon tetrachloride GAD- Glutamate decarboxylase CDK- Cyclin dependent kinase GATA4 -(GATA Binding Protein 4) CK-Cytokeratin GATA6- (GATA Binding Protein 6) CNS- Central Nervous System GCK- Glucokinase CPCSEA- Committee for the purpose of GFP- Green Fluorescent protein control and supervision of experiments on GLP-1- Glucagon-like peptide-1 animals GLUT- Glucose Transporter DAPI- 4’6’-diamidino-2-phenylindole GPx- Glutathione peroxidase Dihydrochloride GSH- Reduced glutathione DCFDA- 2',7' –dichlorofluorescin diacetate GSIS- Glucose-stimulated insulin secretion DM-Diabetes mellitus GTT- Glucose tolerance test DMEM- KO -Dulbecco’s Modified Eagle H & E- Hematoxylin and Eosin Media-Knock out hBMSCs: Human bone marrow-derived DMSO- Dimethyl Sulphoxide mesenchymal stem cell DTZ- Dithizone HGF- Hepatocyte Growth Factor Mitul Vakani. -

Identifying the Role of Wilms Tumor 1 Associated Protein in Cancer Prediction Using Integrative Genomic Analyses
MOLECULAR MEDICINE REPORTS 14: 2823-2831, 2016 Identifying the role of Wilms tumor 1 associated protein in cancer prediction using integrative genomic analyses LI‑SHENG WU1*, JIA-YI QIAN2*, MINGHAI WANG3* and HAIWEI YANG4 1Department of General Surgery, Anhui Provincial Hospital, Anhui Medical University, Hefei, Anhui 230001; 2Department of Breast Surgery, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210029; 3Department of General Surgery, The First Affiliated Yijishan Hospital of Wannan Medical College, Wuhu, Anhui 241002; 4Department of Urology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210029, P.R. China Received August 31, 2015; Accepted June 2, 2016 DOI: 10.3892/mmr.2016.5528 Abstract. The Wilms tumor suppressor, WT1 was first iden- regulatory factor 1, glucocorticoid receptor and peroxisome tified due to its essential role in the normal development of proliferator‑activated receptor γ transcription factor binding the human genitourinary system. Wilms tumor 1 associated sites were identified in the upstream (promoter) region of the protein (WTAP) was subsequently revealed to interact with human WTAP gene, suggesting that these transcription factors WT1 using yeast two‑hybrid screening. The present study may be involved in WTAP functions in tumor formation. identified 44 complete WTAP genes in the genomes of verte- brates, including fish, amphibians, birds and mammals. The Introduction vertebrate WTAP proteins clustered into the primate, rodent and teleost lineages using phylogenetic tree analysis. From The Wilms tumor suppressor gene WT1 was first identified 1,347 available SNPs in the human WTAP gene, 19 were due to its essential role in the normal development of the identified to cause missense mutations. -

SMAD3/Stat3 Signaling Mediates Β-Cell Epithelial-Mesenchymal
2646 Diabetes Volume 66, October 2017 SMAD3/Stat3 Signaling Mediates b-Cell Epithelial- Mesenchymal Transition in Chronic Pancreatitis–Related Diabetes Xiangwei Xiao,1 Shane Fischbach,1 Tina Zhang,1,2 Congde Chen,1 Qingfeng Sheng,1 Ray Zimmerman,1 Sneha Patnaik,1 Joseph Fusco,1 Yungching Ming,1 Ping Guo,1 Chiyo Shiota,1 Krishna Prasadan,1 Nupur Gangopadhyay,1 Sohail Z. Husain,3 Henry Dong,2 and George K. Gittes1 Diabetes 2017;66:2646–2658 | https://doi.org/10.2337/db17-0537 Many patients with chronic pancreatitis develop diabetes Many patients with chronic pancreatitis develop insulinopenia, (chronic pancreatitis–related diabetes [CPRD]) through an glucose intolerance, insulin resistance, and eventually diabetes undetermined mechanism. Here we used long-term par- (2), largely as a result of the intimate proximity of the tial pancreatic duct ligation (PDL) as a model to study CPRD. endocrine pancreas to the exocrine pancreas (3). Moreover, We found that long-term PDL induced significant b-cell de- patients with chronic pancreatitis often develop a fibrotic differentiation, followed by a time-dependent decrease in pancreas with a reduced b-cell mass and have a 15- to b — fi functional -cell mass all speci cally in the ligated tail 16-fold increased risk for pancreatic cancer (4). To date, the portion of the pancreas (PDL-tail). High levels of transform- understanding of the development and pathogenesis of b b ing growth factor 1(TGF 1) were detected in the PDL-tail chronic pancreatitis–relateddiabetes(CPRD)isverylimited. and were mainly produced by M2 macrophages at the In addition, the mechanisms of b-cell loss in CPRD may in early stage and by activated myofibroblasts at the later part be similar to those in type 2 diabetes (T2D) (5,6) and in stage. -

The Role of Epigenomics in Osteoporosis and Osteoporotic Vertebral Fracture
International Journal of Molecular Sciences Review The Role of Epigenomics in Osteoporosis and Osteoporotic Vertebral Fracture Kyoung-Tae Kim 1,2 , Young-Seok Lee 1,3 and Inbo Han 4,* 1 Department of Neurosurgery, School of Medicine, Kyungpook National University, Daegu 41944, Korea; [email protected] (K.-T.K.); [email protected] (Y.-S.L.) 2 Department of Neurosurgery, Kyungpook National University Hospital, Daegu 41944, Korea 3 Department of Neurosurgery, Kyungpook National University Chilgok Hospital, Daegu 41944, Korea 4 Department of Neurosurgery, CHA University School of medicine, CHA Bundang Medical Center, Seongnam-si, Gyeonggi-do 13496, Korea * Correspondence: [email protected]; Tel.: +82-31-780-1924; Fax: +82-31-780-5269 Received: 6 November 2020; Accepted: 8 December 2020; Published: 11 December 2020 Abstract: Osteoporosis is a complex multifactorial condition of the musculoskeletal system. Osteoporosis and osteoporotic vertebral fracture (OVF) are associated with high medical costs and can lead to poor quality of life. Genetic factors are important in determining bone mass and structure, as well as any predisposition for bone degradation and OVF. However, genetic factors are not enough to explain osteoporosis development and OVF occurrence. Epigenetics describes a mechanism for controlling gene expression and cellular processes without altering DNA sequences. The main mechanisms in epigenetics are DNA methylation, histone modifications, and non-coding RNAs (ncRNAs). Recently, alterations in epigenetic mechanisms and their activity have been associated with osteoporosis and OVF. Here, we review emerging evidence that epigenetics contributes to the machinery that can alter DNA structure, gene expression, and cellular differentiation during physiological and pathological bone remodeling. -

Regulation of Target Genes of PAX3−FOXO1 in Alveolar Rhabdomyosarcoma
ANTICANCER RESEARCH 33: 2029-2036 (2013) Regulation of Target Genes of PAX3−FOXO1 in Alveolar Rhabdomyosarcoma EUN HYUN AHN1,2 1Department of Pathology and Laboratory Medicine, School of Medicine, University of Pennsylvania, Philadelphia, PA, U.S.A.; 2Department of Pathology, School of Medicine, University of Washington, Seattle, WA, U.S.A. Abstract. Background: The majority of alveolar major subtypes based on their histological appearance: rhabdomyosarcoma (ARMS) are distinguished through the embryonal rhabdomyosarcoma (ERMS) and alveolar paired box 3−forkhead box protein O1 (PAX3−FOXO1) rhabdomyosarcoma (ARMS) (1). ARMS has a higher fusion oncoprotein, being generated by a 2;13 chromosomal frequency of metastases at the initial diagnosis than ERMS, translocation. This fusion-positive ARMS is the most commonly conferring a poorer prognosis than ERMS (2, 3). clinically difficult type of rhabdomyosarcoma. The present A common characteristic of ERMS is a loss of study characterized four genes [gremlin 1 (GREM1), death- heterozygosity at 11p15, however ERMS has not been associated protein kinase-1 (DAPK1), myogenic reported to exhibit a diagnostic genetic alteration. In contrast, differentiation-1 (MYOD1), and hairy/enhancer-of-split chromosomal translocation is frequently observed for ARMS related with YRPW motif-1 (HEY1)] as targets of (4, 5). The translocation t(2;13)(q35;q14) generating the PAX3−FOXO1. Materials and Methods: The expression of paired box 3−forkhead box protein O1 (PAX3−FOXO1) gene the four genes, PAX3−FOXO1, and v-myc myelocytomatosis fusion was found to occur in 55% of ARMS cases, while the viral-related oncogene, neuroblastoma-derived (avian) translocation t(1;13)(q36;q14) generating the paired box (MYCN) was determined in various ARMS cell models and 7−forkhead box protein O1 (PAX7−FOXO1) gene fusion primary tumors. -
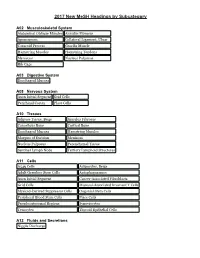
2017 New Mesh Headings by Subcategory
2017 New MeSH Headings by Subcategory A02 Musculoskeletal System Abdominal Oblique Muscles Annulus Fibrosus Aponeurosis Collateral Ligament, Ulnar Coracoid Process Gracilis Muscle Hamstring Muscles Hamstring Tendons Meniscus Nucleus Pulposus Rib Cage A03 Digestive System Esophageal Mucosa A08 Nervous System Axon Initial Segment Grid Cells Perirhinal Cortex Place Cells A10 Tissues Adipose Tissue, Beige Annulus Fibrosus Cancellous Bone Cortical Bone Esophageal Mucosa Hamstring Muscles Margins of Excision Meniscus Nucleus Pulposus Parenchymal Tissue Sentinel Lymph Node Tertiary Lymphoid Structures A11 Cells A549 Cells Adipocytes, Beige Adult Germline Stem Cells Autophagosomes Axon Initial Segment Cancer-Associated Fibroblasts Grid Cells Mucosal-Associated Invariant T Cells Myeloid-Derived Suppressor Cells Oogonial Stem Cells Peripheral Blood Stem Cells Place Cells Pseudoautosomal Regions Synoviocytes Tenocytes Thyroid Epithelial Cells A12 Fluids and Secretions Nipple Discharge 2017 New MeSH Headings by Subcategory A13 Animal Structures Gizzard, Non-avian A15 Hemic and Immune Systems Mucosal-Associated Invariant T Cells Sentinel Lymph Node Tertiary Lymphoid Structures A16 Embryonic Structures Gubernaculum B01 Eukaryota Amaryllidaceae Asparagaceae Asparagales Camelidae Colchicaceae Endamoeba histolytica Gentianales Hypoxidaceae Liliales Loteae Melanthiaceae Ocimum sanctum Pogostemon Psacalium Saccharomyces boulardii Vigna Wolfiporia Xanthorrhoeaceae 2017 New MeSH Headings by Subcategory B03 Bacteria Aeromonas veronii Bacillus amyloliquefaciens -

VE-Cadherin–Mediated Epigenetic Regulation of Endothelial Gene Expression
Molecular Medicine VE-Cadherin–Mediated Epigenetic Regulation of Endothelial Gene Expression Marco F. Morini,* Costanza Giampietro,* Monica Corada, Federica Pisati, Elisa Lavarone, Sara I. Cunha, Lei L. Conze, Nicola O’Reilly, Dhira Joshi, Svend Kjaer, Roger George, Emma Nye, Anqi Ma, Jian Jin, Richard Mitter, Michela Lupia, Ugo Cavallaro, Diego Pasini, Dinis P. Calado, Elisabetta Dejana, Andrea Taddei Rationale: The mechanistic foundation of vascular maturation is still largely unknown. Several human pathologies are characterized by deregulated angiogenesis and unstable blood vessels. Solid tumors, for instance, get their nourishment from newly formed structurally abnormal vessels which present wide and irregular interendothelial junctions. Expression and clustering of the main endothelial-specific adherens junction protein, VEC (vascular endothelial cadherin), upregulate genes with key roles in endothelial differentiation and stability. Downloaded from Objective: We aim at understanding the molecular mechanisms through which VEC triggers the expression of a set of genes involved in endothelial differentiation and vascular stabilization. Methods and Results: We compared a VEC-null cell line with the same line reconstituted with VEC wild-type cDNA. VEC expression and clustering upregulated endothelial-specific genes with key roles in vascular stabilization including claudin-5, vascular endothelial-protein tyrosine phosphatase (VE-PTP), and von Willebrand factor (vWf). http://circres.ahajournals.org/ Mechanistically, VEC exerts this effect by inhibiting polycomb protein activity on the specific gene promoters. This is achieved by preventing nuclear translocation of FoxO1 (Forkhead box protein O1) and β-catenin, which contribute to PRC2 (polycomb repressive complex-2) binding to promoter regions of claudin-5, VE-PTP, and vWf. VEC/β-catenin complex also sequesters a core subunit of PRC2 (Ezh2 [enhancer of zeste homolog 2]) at the cell membrane, preventing its nuclear translocation. -

Expression Analysis of FGF/FGFR and FOX Family Proteins in Mucosal Tissue Obtained from Orofacial Cleft-Affected Children
biology Article Expression Analysis of FGF/FGFR and FOX Family Proteins in Mucosal Tissue Obtained from Orofacial Cleft-Affected Children Mara¯ Pilmane , Nityanand Jain * and Zane Vitenberga-Verza Department of Morphology, Institute of Anatomy and Anthropology, Riga Stradinš University, LV-1007 Riga, Latvia; [email protected] (M.P.); [email protected] (Z.V.-V.) * Correspondence: [email protected]; Tel.: +371-67320862 Simple Summary: Craniofacial development is an intricate and delicate process in normal embryo- genesis requiring spatiotemporal release of various mediators/proteins that provide communication between different cell/tissue types, like epithelial cells, connective tissue, and endothelial cells. If this sequence is impaired or blocked due to genetic or environmental factors, it can lead to clefting. A cleft is an abnormal space or gap in the upper lip, alveolus, or palate that occurs due to failure of completion of fusion processes in the midline during facial development. Previous studies have identified various genetic factors (genes) that can lead to clefting. The most promising candidates amongst them are FGF/FGFR (fibroblast growth factor/FGF receptor) signaling genes and FOX (fork- head box protein) genes. We investigated the expression of these genes in tissue material obtained from cleft-affected patients. Our results indicate that these genes profoundly affect the pathogenesis and manifestation of clefts, especially by enhancing local site inflammation and fibrosis. Further, they play a vital role in angiogenesis, apoptosis, and cell proliferation. Citation: Pilmane, M.; Jain, N.; Vitenberga-Verza, Z. Expression Abstract: Orofacial clefts affect hundreds of thousands of children worldwide annually and are Analysis of FGF/FGFR and FOX usually corrected by a series of surgeries extending to childhood. -

Mirna Clusters with Up-Regulated Expression in Colorectal Cancer
cancers Review miRNA Clusters with Up-Regulated Expression in Colorectal Cancer Paulína Pidíková and Iveta Herichová * Department of Animal Physiology and Ethology, Faculty of Natural Sciences, Comenius University in Bratislava, Ilkoviˇcova6, 842 15 Bratislava, Slovakia; [email protected] * Correspondence: [email protected]; Tel.: +421-602-96-572 Simple Summary: As miRNAs show the capacity to be used as CRC biomarkers, we analysed exper- imentally validated data about frequently up-regulated miRNA clusters in CRC tissue. We identified 15 clusters that showed increased expression in CRC: miR-106a/363, miR-106b/93/25, miR-17/92a-1, miR-181a-1/181b-1, miR-181a-2/181b-2, miR-181c/181d, miR-183/96/182, miR-191/425, miR-200c/141, miR-203a/203b, miR-222/221, mir-23a/27a/24-2, mir-29b-1/29a, mir-301b/130b and mir-452/224. Cluster positions in the genome are intronic or intergenic. Most clusters are regu- lated by several transcription factors, and by long non-coding RNAs. In some cases, co-expression of miRNA with other cluster members or host gene has been proven. miRNA expression patterns in cancer tissue, blood and faeces were compared. The members of the selected clusters target 181 genes. Their functions and corresponding pathways were revealed with the use of Panther analysis. Clusters miR-17/92a-1, miR-106a/363, miR-106b/93/25 and miR-183/96/182 showed the strongest association with metastasis occurrence and poor patient survival, implicating them as the most promising targets of translational research. Abstract: Colorectal cancer (CRC) is one of the most common malignancies in Europe and North Citation: Pidíková, P.; Herichová, I. -

Three Novel Genes Predicting Depression in Gene X Environment Interactions Are Identified Using Cross-Species and Cross-Tissues Transcriptomic and Mirnomic Analyses
Molecular Psychiatry (2018) 23:2192–2208 https://doi.org/10.1038/s41380-017-0002-4 ARTICLE FoxO1, A2M, and TGF-β1: three novel genes predicting depression in gene X environment interactions are identified using cross-species and cross-tissues transcriptomic and miRNomic analyses 1,2 2 2 3 4 1 Annamaria Cattaneo ● Nadia Cattane ● Chiara Malpighi ● Darina Czamara ● Anna Suarez ● Nicole Mariani ● 5,6,7,8 9 5,10,11 4,6,7,12 1 13 Eero Kajantie ● Alessia Luoni ● Johan G. Eriksson ● Jari Lahti ● Valeria Mondelli ● Paola Dazzan ● 4 3,14 9 1 Katri Räikkönen ● Elisabeth B. Binder ● Marco A. Riva ● Carmine M. Pariante Received: 9 December 2016 / Revised: 9 September 2017 / Accepted: 16 October 2017 / Published online: 4 January 2018 © The Author(s) 2017. This article is published with open access Abstract To date, gene-environment (GxE) interaction studies in depression have been limited to hypothesis-based candidate genes, since genome-wide (GWAS)-based GxE interaction studies would require enormous datasets with genetics, environmental, and clinical variables. We used a novel, cross-species and cross-tissues “omics” approach to identify genes predicting depression in response to stress in GxE interactions. We integrated the transcriptome and miRNome profiles 1234567890 from the hippocampus of adult rats exposed to prenatal stress (PNS) with transcriptome data obtained from blood mRNA of adult humans exposed to early life trauma, using a stringent statistical analyses pathway. Network analysis of the integrated gene lists identified the Forkhead box protein O1 (FoxO1), Alpha-2-Macroglobulin (A2M), and Transforming Growth Factor Beta 1 (TGF-β1) as candidates to be tested for GxE interactions, in two GWAS samples of adults either with a range of childhood traumatic experiences (Grady Study Project, Atlanta, USA) or with separation from parents in childhood only (Helsinki Birth Cohort Study, Finland). -

Narita Et Al., NRMCB 2019 AAM
Functions and mechanisms of non-histone protein acetylation Narita, Takeo; Weinert, Brian T; Choudhary, Chunaram Published in: Nature Reviews. Molecular Cell Biology DOI: 10.1038/s41580-018-0081-3 Publication date: 2019 Document version Peer reviewed version Citation for published version (APA): Narita, T., Weinert, B. T., & Choudhary, C. (2019). Functions and mechanisms of non-histone protein acetylation. Nature Reviews. Molecular Cell Biology, 20, 156-174. https://doi.org/10.1038/s41580-018-0081-3 Download date: 26. sep.. 2021 Functions and mechanisms of non-histone protein acetylation Takeo Narita, Brian T. Weinert, Chunaram Choudhary* The Novo Nordisk Foundation Center for Protein Research, Faculty of Health Sciences, University of Copenhagen, Blegdamsvej 3, 2200 Copenhagen, Denmark *Correspondence should be addressed to: CC, [email protected] Abstract | N-ε-lysine acetylation was discovered more than half a century ago as a posttranslational modification of histones and has been extensively studied in the context of transcription regulation. In the past decade, proteomic analyses have revealed that non-histone proteins are frequently acetylated and constitute a major portion of the acetylome in mammalian cells. Indeed, non-histone protein acetylation is involved in key cellular processes relevant to physiology and disease, such as gene transcription, DNA damage repair, cell division, signal transduction, protein folding, autophagy and metabolism. Acetylation affects protein functions through diverse mechanisms, including by regulating protein stability, enzymatic activity, subcellular localization, cross-talk with other posttranslational modifications, and by controlling protein–protein and protein–DNA interactions. In this Review, we discuss recent progress in our understanding of the scope, functional diversity and mechanisms of non-histone protein acetylation. -

Exploring the Genetic Conception of Obesity Via the Dual Role of Foxo
International Journal of Molecular Sciences Review Exploring the Genetic Conception of Obesity via the Dual Role of FoxO Tapan Behl 1,*, Ishnoor Kaur 1, Aayush Sehgal 1, Sukhbir Singh 1, Gokhan Zengin 2, Nicoleta Negrut 3 , Delia Carmen Nistor-Cseppento 3, Flavia Maria Pavel 4,†, Raluca Anca Corb Aron 4,† and Simona Bungau 5,* 1 Department of Pharmacology, Chitkara College of Pharmacy, Chitkara University, Punjab 140401, India; [email protected] (I.K.); [email protected] (A.S.); [email protected] (S.S.) 2 Department of Biology, Faculty of Science, Selcuk University Campus, Konya 42130, Turkey; [email protected] 3 Department of Psycho-Neuroscience and Recovery, Faculty of Medicine and Pharmacy, University of Oradea, 410073 Oradea, Romania; [email protected] (N.N.); [email protected] (D.C.N.-C.) 4 Department of Preclinical Disciplines, Faculty of Medicine and Pharmacy, University of Oradea, 410073 Oradea, Romania; fl[email protected] (F.M.P.); [email protected] (R.A.C.A.) 5 Department of Pharmacy, Faculty of Medicine and Pharmacy, University of Oradea, 410028 Oradea, Romania * Correspondence: [email protected] (T.B.); [email protected] (S.B.); Tel.: +40-726-776-588 (S.B.) † These authors contributed equally to the first author. Abstract: Obesity or overweight are not superficial problems, constituting a pressing issue. The obesity index has almost tripled since 1975, which is an alarming state. Most of the individuals are currently becoming overweight or have inappropriate body mass index (BMI) conditions. Obesity is characterized by increased fat accumulation and thus poses a higher health risk. There is increased size and volume of fat cells in the body, which usually accounts for obesity.