Narita Et Al., NRMCB 2019 AAM
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Abbreviation
Exploring and Potentiating Human Bone Marrow Derived Mesenchymal Stem Cells [hBMSCs] and Differentiated Islets for Effective Diabetes Therapy ABBREVIATION LIST OF ABBREVIATION AF555-Alexa Fluor 555 EBs- Embryoid bodies AGE- advanced glycosylation end-product EDTA- Ethylenediaminetetraacetic acid aPKC- Atypical protein kinase C EGF-Epidermal growth factor ANOVA-Analysis of variance EGFR- epidermal growth factor receptor Arx- Aristaless-related homeobox gene EL- Enicostemma littorale ATP- Adenosine triphosphate ELISA- Enzyme linked immunosorbent bHLH- Basic helix–loop–helix assay BM- Bone marrow EMT - epithelial–mesenchymal transition BMC- Bioactive molecules cocktail ESCs- Embryonic stem cells BMP-bone morphogenic protein FACS- Fluorescent activated cell sorter BSA- Bovine serum albumin FBS- Fetal Bovine Serum cAMP- Cyclic Adenosine monophosphate FDA- Food and Drug Administration CaCl2 –Calcium chloride FGF- Fibroblast growth factor CD-Cluster of differentiation FITC- Fluorescein isothiocyanate cDNA –Complementary DNA FOXO1-Forkhead box protein O1 ChIP-Chromatin immunoprecipitation FOXA2 - Forkhead box protein A2 CCl4- Carbon tetrachloride GAD- Glutamate decarboxylase CDK- Cyclin dependent kinase GATA4 -(GATA Binding Protein 4) CK-Cytokeratin GATA6- (GATA Binding Protein 6) CNS- Central Nervous System GCK- Glucokinase CPCSEA- Committee for the purpose of GFP- Green Fluorescent protein control and supervision of experiments on GLP-1- Glucagon-like peptide-1 animals GLUT- Glucose Transporter DAPI- 4’6’-diamidino-2-phenylindole GPx- Glutathione peroxidase Dihydrochloride GSH- Reduced glutathione DCFDA- 2',7' –dichlorofluorescin diacetate GSIS- Glucose-stimulated insulin secretion DM-Diabetes mellitus GTT- Glucose tolerance test DMEM- KO -Dulbecco’s Modified Eagle H & E- Hematoxylin and Eosin Media-Knock out hBMSCs: Human bone marrow-derived DMSO- Dimethyl Sulphoxide mesenchymal stem cell DTZ- Dithizone HGF- Hepatocyte Growth Factor Mitul Vakani. -

Identifying the Role of Wilms Tumor 1 Associated Protein in Cancer Prediction Using Integrative Genomic Analyses
MOLECULAR MEDICINE REPORTS 14: 2823-2831, 2016 Identifying the role of Wilms tumor 1 associated protein in cancer prediction using integrative genomic analyses LI‑SHENG WU1*, JIA-YI QIAN2*, MINGHAI WANG3* and HAIWEI YANG4 1Department of General Surgery, Anhui Provincial Hospital, Anhui Medical University, Hefei, Anhui 230001; 2Department of Breast Surgery, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210029; 3Department of General Surgery, The First Affiliated Yijishan Hospital of Wannan Medical College, Wuhu, Anhui 241002; 4Department of Urology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210029, P.R. China Received August 31, 2015; Accepted June 2, 2016 DOI: 10.3892/mmr.2016.5528 Abstract. The Wilms tumor suppressor, WT1 was first iden- regulatory factor 1, glucocorticoid receptor and peroxisome tified due to its essential role in the normal development of proliferator‑activated receptor γ transcription factor binding the human genitourinary system. Wilms tumor 1 associated sites were identified in the upstream (promoter) region of the protein (WTAP) was subsequently revealed to interact with human WTAP gene, suggesting that these transcription factors WT1 using yeast two‑hybrid screening. The present study may be involved in WTAP functions in tumor formation. identified 44 complete WTAP genes in the genomes of verte- brates, including fish, amphibians, birds and mammals. The Introduction vertebrate WTAP proteins clustered into the primate, rodent and teleost lineages using phylogenetic tree analysis. From The Wilms tumor suppressor gene WT1 was first identified 1,347 available SNPs in the human WTAP gene, 19 were due to its essential role in the normal development of the identified to cause missense mutations. -

SMAD3/Stat3 Signaling Mediates Β-Cell Epithelial-Mesenchymal
2646 Diabetes Volume 66, October 2017 SMAD3/Stat3 Signaling Mediates b-Cell Epithelial- Mesenchymal Transition in Chronic Pancreatitis–Related Diabetes Xiangwei Xiao,1 Shane Fischbach,1 Tina Zhang,1,2 Congde Chen,1 Qingfeng Sheng,1 Ray Zimmerman,1 Sneha Patnaik,1 Joseph Fusco,1 Yungching Ming,1 Ping Guo,1 Chiyo Shiota,1 Krishna Prasadan,1 Nupur Gangopadhyay,1 Sohail Z. Husain,3 Henry Dong,2 and George K. Gittes1 Diabetes 2017;66:2646–2658 | https://doi.org/10.2337/db17-0537 Many patients with chronic pancreatitis develop diabetes Many patients with chronic pancreatitis develop insulinopenia, (chronic pancreatitis–related diabetes [CPRD]) through an glucose intolerance, insulin resistance, and eventually diabetes undetermined mechanism. Here we used long-term par- (2), largely as a result of the intimate proximity of the tial pancreatic duct ligation (PDL) as a model to study CPRD. endocrine pancreas to the exocrine pancreas (3). Moreover, We found that long-term PDL induced significant b-cell de- patients with chronic pancreatitis often develop a fibrotic differentiation, followed by a time-dependent decrease in pancreas with a reduced b-cell mass and have a 15- to b — fi functional -cell mass all speci cally in the ligated tail 16-fold increased risk for pancreatic cancer (4). To date, the portion of the pancreas (PDL-tail). High levels of transform- understanding of the development and pathogenesis of b b ing growth factor 1(TGF 1) were detected in the PDL-tail chronic pancreatitis–relateddiabetes(CPRD)isverylimited. and were mainly produced by M2 macrophages at the In addition, the mechanisms of b-cell loss in CPRD may in early stage and by activated myofibroblasts at the later part be similar to those in type 2 diabetes (T2D) (5,6) and in stage. -

Computational Modeling of Lysine Post-Translational Modification: an Overview Md
c and S eti ys h te nt m y s S B Hasan MM et al., Curr Synthetic Sys Biol 2018, 6:1 t i n o e l Current Synthetic and o r r g DOI: 10.4172/2332-0737.1000137 u y C ISSN: 2332-0737 Systems Biology CommentaryResearch Article OpenOpen Access Access Computational Modeling of Lysine Post-Translational Modification: An Overview Md. Mehedi Hasan 1*, Mst. Shamima Khatun2, and Hiroyuki Kurata1,3 1Department of Bioscience and Bioinformatics, Kyushu Institute of Technology, 680-4 Kawazu, Iizuka, Fukuoka 820-8502, Japan 2Department of Statistics, Laboratory of Bioinformatics, Rajshahi University-6205, Bangladesh 3Biomedical Informatics R&D Center, Kyushu Institute of Technology, 680-4 Kawazu, Iizuka, Fukuoka 820-8502, Japan Commentary hot spot for PTMs, and a number of protein lysine modifications could occur in both histone and non-histone proteins [11,12]. For instance, Living organisms have a magnificent ordered and complex lysine methylation in non-histone proteins can regulate the protein structure. In regulating the cellular functions, post-translational activity and protein structure stability [13]. In 2004, the Nobel Prize in modifications (PTMs) are critical molecular measures. They alter Chemistry was awarded jointly to Aaron Ciechanover, Avram Hershko protein conformation, modulating their activity, stability and and Irwin Rose for the discovery of lysine ubiquitin-mediated protein localization. Up to date, more than 300 types of PTMs are experimentally degradation [14]. discovered in vivo and in vitro pathways [1,2]. Major and common PTMs are methylation, ubiquitination, succinylation, phosphorylation, Moreover, in biological process, lysine can be modified by the glycosylation, acetylation, and sumoylation. -

Loss of Conserved Ubiquitylation Sites in Conserved Proteins During Human Evolution
INTERNATIONAL JOURNAL OF MOleCular meDICine 42: 2203-2212, 2018 Loss of conserved ubiquitylation sites in conserved proteins during human evolution DONGBIN PARK, CHUL JUN GOH, HYEIN KIM, JI SEOK LEE and YOONSOO HAHN Department of Life Science, Chung‑Ang University, Seoul 06974, Republic of Korea Received January 30, 2018; Accepted July 6, 2018 DOI: 10.3892/ijmm.2018.3772 Abstract. Ubiquitylation of lysine residues in proteins serves Introduction a pivotal role in the efficient removal of misfolded or unused proteins and in the control of various regulatory pathways Ubiquitylation, in which the highly conserved 76‑residue poly- by monitoring protein activity that may lead to protein peptide ubiquitin is covalently attached to a lysine residue of degradation. The loss of ubiquitylated lysines may affect substrate proteins, mediates the targeted destruction of ubiq- the ubiquitin‑mediated regulatory network and result in the uitylated proteins by the ubiquitin‑proteasome system (1‑4). emergence of novel phenotypes. The present study analyzed The ubiquitin‑mediated protein degradation pathway serves a mouse ubiquitylation data and orthologous proteins from crucial role in the efficient and specific removal of misfolded 62 mammals to identify 193 conserved ubiquitylation sites from proteins and certain key regulatory proteins (5,6). Ubiquitin 169 proteins that were lost in the Euarchonta lineage leading and other ubiquitin‑like proteins, including autophagy‑related to humans. A total of 8 proteins, including betaine homo- protein 8, Ubiquitin‑like -

Metabolomics-Assisted Proteomics Identifies Succinylation and SIRT5 As Important Regulators of Cardiac Function
Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function Sushabhan Sadhukhana, Xiaojing Liub,c,d, Dongryeol Ryue, Ornella D. Nelsona, John A. Stupinskif, Zhi Lia, Wei Cheng, Sheng Zhangg, Robert S. Weissf, Jason W. Locasaleb,c,d, Johan Auwerxe,1, and Hening Lina,h,1 aDepartment of Chemistry and Chemical Biology, Cornell University, Ithaca, NY 14853; bDuke Cancer Institute, Duke University School of Medicine, Durham, NC 27710; cDuke Molecular Physiology Institute, Duke University School of Medicine, Durham, NC 27710; dDepartment of Pharmacology and Cancer Biology, Duke University School of Medicine, Durham, NC 27710; eLaboratory of Integrative and Systems Physiology, School of Life Sciences, École Polytechnique Fédérale de Lausanne, 1015 Lausanne, Switzerland; fDepartment of Biomedical Sciences, Cornell University, Ithaca, NY 14853; gProteomics & Mass Spectrometry Facility, Institute of Biotechnology, Cornell University, Ithaca, NY 14853; and hHoward Hughes Medical Institute, Cornell University, Ithaca, NY 14853 Edited by Kevan M. Shokat, University of California, San Francisco, CA, and approved March 9, 2016 (received for review October 7, 2015) Cellular metabolites, such as acyl-CoA, can modify proteins, leading SIRT4 and SIRT5 have very weak deacetylase activities (14). SIRT5 to protein posttranslational modifications (PTMs). One such PTM is possesses unique enzymatic activity on hydrolyzing negatively lysine succinylation, which is regulated by sirtuin 5 (SIRT5). Although charged -

SUMO and Transcriptional Regulation: the Lessons of Large-Scale Proteomic, Modifomic and Genomic Studies
molecules Review SUMO and Transcriptional Regulation: The Lessons of Large-Scale Proteomic, Modifomic and Genomic Studies Mathias Boulanger 1,2 , Mehuli Chakraborty 1,2, Denis Tempé 1,2, Marc Piechaczyk 1,2,* and Guillaume Bossis 1,2,* 1 Institut de Génétique Moléculaire de Montpellier (IGMM), University of Montpellier, CNRS, Montpellier, France; [email protected] (M.B.); [email protected] (M.C.); [email protected] (D.T.) 2 Equipe Labellisée Ligue Contre le Cancer, Paris, France * Correspondence: [email protected] (M.P.); [email protected] (G.B.) Abstract: One major role of the eukaryotic peptidic post-translational modifier SUMO in the cell is transcriptional control. This occurs via modification of virtually all classes of transcriptional actors, which include transcription factors, transcriptional coregulators, diverse chromatin components, as well as Pol I-, Pol II- and Pol III transcriptional machineries and their regulators. For many years, the role of SUMOylation has essentially been studied on individual proteins, or small groups of proteins, principally dealing with Pol II-mediated transcription. This provided only a fragmentary view of how SUMOylation controls transcription. The recent advent of large-scale proteomic, modifomic and genomic studies has however considerably refined our perception of the part played by SUMO in gene expression control. We review here these developments and the new concepts they are at the origin of, together with the limitations of our knowledge. How they illuminate the SUMO-dependent Citation: Boulanger, M.; transcriptional mechanisms that have been characterized thus far and how they impact our view of Chakraborty, M.; Tempé, D.; SUMO-dependent chromatin organization are also considered. -

The Role of Epigenomics in Osteoporosis and Osteoporotic Vertebral Fracture
International Journal of Molecular Sciences Review The Role of Epigenomics in Osteoporosis and Osteoporotic Vertebral Fracture Kyoung-Tae Kim 1,2 , Young-Seok Lee 1,3 and Inbo Han 4,* 1 Department of Neurosurgery, School of Medicine, Kyungpook National University, Daegu 41944, Korea; [email protected] (K.-T.K.); [email protected] (Y.-S.L.) 2 Department of Neurosurgery, Kyungpook National University Hospital, Daegu 41944, Korea 3 Department of Neurosurgery, Kyungpook National University Chilgok Hospital, Daegu 41944, Korea 4 Department of Neurosurgery, CHA University School of medicine, CHA Bundang Medical Center, Seongnam-si, Gyeonggi-do 13496, Korea * Correspondence: [email protected]; Tel.: +82-31-780-1924; Fax: +82-31-780-5269 Received: 6 November 2020; Accepted: 8 December 2020; Published: 11 December 2020 Abstract: Osteoporosis is a complex multifactorial condition of the musculoskeletal system. Osteoporosis and osteoporotic vertebral fracture (OVF) are associated with high medical costs and can lead to poor quality of life. Genetic factors are important in determining bone mass and structure, as well as any predisposition for bone degradation and OVF. However, genetic factors are not enough to explain osteoporosis development and OVF occurrence. Epigenetics describes a mechanism for controlling gene expression and cellular processes without altering DNA sequences. The main mechanisms in epigenetics are DNA methylation, histone modifications, and non-coding RNAs (ncRNAs). Recently, alterations in epigenetic mechanisms and their activity have been associated with osteoporosis and OVF. Here, we review emerging evidence that epigenetics contributes to the machinery that can alter DNA structure, gene expression, and cellular differentiation during physiological and pathological bone remodeling. -

Ubiquitin Modifications
npg Cell Research (2016) 26:399-422. REVIEW www.nature.com/cr Ubiquitin modifications Kirby N Swatek1, David Komander1 1Medical Research Council Laboratory of Molecular Biology, Francis Crick Avenue, Cambridge, CB2 0QH, UK Protein ubiquitination is a dynamic multifaceted post-translational modification involved in nearly all aspects of eukaryotic biology. Once attached to a substrate, the 76-amino acid protein ubiquitin is subjected to further modi- fications, creating a multitude of distinct signals with distinct cellular outcomes, referred to as the ‘ubiquitin code’. Ubiquitin can be ubiquitinated on seven lysine (Lys) residues or on the N-terminus, leading to polyubiquitin chains that can encompass complex topologies. Alternatively or in addition, ubiquitin Lys residues can be modified by ubiq- uitin-like molecules (such as SUMO or NEDD8). Finally, ubiquitin can also be acetylated on Lys, or phosphorylated on Ser, Thr or Tyr residues, and each modification has the potential to dramatically alter the signaling outcome. While the number of distinctly modified ubiquitin species in cells is mind-boggling, much progress has been made to characterize the roles of distinct ubiquitin modifications, and many enzymes and receptors have been identified that create, recognize or remove these ubiquitin modifications. We here provide an overview of the various ubiquitin modifications present in cells, and highlight recent progress on ubiquitin chain biology. We then discuss the recent findings in the field of ubiquitin acetylation and phosphorylation, with a focus on Ser65-phosphorylation and its role in mitophagy and Parkin activation. Keywords: ubiquitin; proteasomal degradation; phosphorylation; post-translational modification; Parkin Cell Research (2016) 26:399-422. -

SUCLA2 Mutations Cause Global Protein Succinylation Contributing To
ARTICLE https://doi.org/10.1038/s41467-020-19743-4 OPEN SUCLA2 mutations cause global protein succinylation contributing to the pathomechanism of a hereditary mitochondrial disease ✉ Philipp Gut 1,2,17 , Sanna Matilainen3,17, Jesse G. Meyer4,14,17, Pieti Pällijeff3, Joy Richard2, Christopher J. Carroll5, Liliya Euro 3, Christopher B. Jackson 3, Pirjo Isohanni3,6, Berge A. Minassian7,8, Reem A. Alkhater9, Elsebet Østergaard10, Gabriele Civiletto2, Alice Parisi2, Jonathan Thevenet2, Matthew J. Rardin4,15, Wenjuan He1, Yuya Nishida1, John C. Newman 1, Xiaojing Liu11,16, Stefan Christen2, ✉ ✉ Sofia Moco 2, Jason W. Locasale 11, Birgit Schilling 4,18 , Anu Suomalainen 3,12,13,18 & 1234567890():,; ✉ Eric Verdin 1,4,18 Mitochondrial acyl-coenzyme A species are emerging as important sources of protein modification and damage. Succinyl-CoA ligase (SCL) deficiency causes a mitochondrial encephalomyopathy of unknown pathomechanism. Here, we show that succinyl-CoA accumulates in cells derived from patients with recessive mutations in the tricarboxylic acid cycle (TCA) gene succinyl-CoA ligase subunit-β (SUCLA2), causing global protein hyper- succinylation. Using mass spectrometry, we quantify nearly 1,000 protein succinylation sites on 366 proteins from patient-derived fibroblasts and myotubes. Interestingly, hyper- succinylated proteins are distributed across cellular compartments, and many are known targets of the (NAD+)-dependent desuccinylase SIRT5. To test the contribution of hyper- succinylation to disease progression, we develop a zebrafish model of the SCL deficiency and find that SIRT5 gain-of-function reduces global protein succinylation and improves survival. Thus, increased succinyl-CoA levels contribute to the pathology of SCL deficiency through post-translational modifications. -

Regulation of Target Genes of PAX3−FOXO1 in Alveolar Rhabdomyosarcoma
ANTICANCER RESEARCH 33: 2029-2036 (2013) Regulation of Target Genes of PAX3−FOXO1 in Alveolar Rhabdomyosarcoma EUN HYUN AHN1,2 1Department of Pathology and Laboratory Medicine, School of Medicine, University of Pennsylvania, Philadelphia, PA, U.S.A.; 2Department of Pathology, School of Medicine, University of Washington, Seattle, WA, U.S.A. Abstract. Background: The majority of alveolar major subtypes based on their histological appearance: rhabdomyosarcoma (ARMS) are distinguished through the embryonal rhabdomyosarcoma (ERMS) and alveolar paired box 3−forkhead box protein O1 (PAX3−FOXO1) rhabdomyosarcoma (ARMS) (1). ARMS has a higher fusion oncoprotein, being generated by a 2;13 chromosomal frequency of metastases at the initial diagnosis than ERMS, translocation. This fusion-positive ARMS is the most commonly conferring a poorer prognosis than ERMS (2, 3). clinically difficult type of rhabdomyosarcoma. The present A common characteristic of ERMS is a loss of study characterized four genes [gremlin 1 (GREM1), death- heterozygosity at 11p15, however ERMS has not been associated protein kinase-1 (DAPK1), myogenic reported to exhibit a diagnostic genetic alteration. In contrast, differentiation-1 (MYOD1), and hairy/enhancer-of-split chromosomal translocation is frequently observed for ARMS related with YRPW motif-1 (HEY1)] as targets of (4, 5). The translocation t(2;13)(q35;q14) generating the PAX3−FOXO1. Materials and Methods: The expression of paired box 3−forkhead box protein O1 (PAX3−FOXO1) gene the four genes, PAX3−FOXO1, and v-myc myelocytomatosis fusion was found to occur in 55% of ARMS cases, while the viral-related oncogene, neuroblastoma-derived (avian) translocation t(1;13)(q36;q14) generating the paired box (MYCN) was determined in various ARMS cell models and 7−forkhead box protein O1 (PAX7−FOXO1) gene fusion primary tumors. -
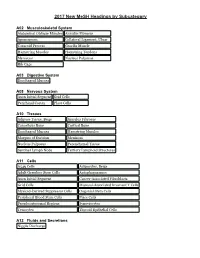
2017 New Mesh Headings by Subcategory
2017 New MeSH Headings by Subcategory A02 Musculoskeletal System Abdominal Oblique Muscles Annulus Fibrosus Aponeurosis Collateral Ligament, Ulnar Coracoid Process Gracilis Muscle Hamstring Muscles Hamstring Tendons Meniscus Nucleus Pulposus Rib Cage A03 Digestive System Esophageal Mucosa A08 Nervous System Axon Initial Segment Grid Cells Perirhinal Cortex Place Cells A10 Tissues Adipose Tissue, Beige Annulus Fibrosus Cancellous Bone Cortical Bone Esophageal Mucosa Hamstring Muscles Margins of Excision Meniscus Nucleus Pulposus Parenchymal Tissue Sentinel Lymph Node Tertiary Lymphoid Structures A11 Cells A549 Cells Adipocytes, Beige Adult Germline Stem Cells Autophagosomes Axon Initial Segment Cancer-Associated Fibroblasts Grid Cells Mucosal-Associated Invariant T Cells Myeloid-Derived Suppressor Cells Oogonial Stem Cells Peripheral Blood Stem Cells Place Cells Pseudoautosomal Regions Synoviocytes Tenocytes Thyroid Epithelial Cells A12 Fluids and Secretions Nipple Discharge 2017 New MeSH Headings by Subcategory A13 Animal Structures Gizzard, Non-avian A15 Hemic and Immune Systems Mucosal-Associated Invariant T Cells Sentinel Lymph Node Tertiary Lymphoid Structures A16 Embryonic Structures Gubernaculum B01 Eukaryota Amaryllidaceae Asparagaceae Asparagales Camelidae Colchicaceae Endamoeba histolytica Gentianales Hypoxidaceae Liliales Loteae Melanthiaceae Ocimum sanctum Pogostemon Psacalium Saccharomyces boulardii Vigna Wolfiporia Xanthorrhoeaceae 2017 New MeSH Headings by Subcategory B03 Bacteria Aeromonas veronii Bacillus amyloliquefaciens