Visual Modelling Suggests a Weak Relationship Between the Evolution of Ultraviolet Vision and Plumage Colouration in Birds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
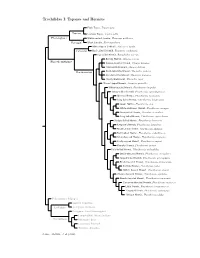
Topazes and Hermits
Trochilidae I: Topazes and Hermits Fiery Topaz, Topaza pyra Topazini Crimson Topaz, Topaza pella Florisuginae White-necked Jacobin, Florisuga mellivora Florisugini Black Jacobin, Florisuga fusca White-tipped Sicklebill, Eutoxeres aquila Eutoxerini Buff-tailed Sicklebill, Eutoxeres condamini Saw-billed Hermit, Ramphodon naevius Bronzy Hermit, Glaucis aeneus Phaethornithinae Rufous-breasted Hermit, Glaucis hirsutus ?Hook-billed Hermit, Glaucis dohrnii Threnetes ruckeri Phaethornithini Band-tailed Barbthroat, Pale-tailed Barbthroat, Threnetes leucurus ?Sooty Barbthroat, Threnetes niger ?Broad-tipped Hermit, Anopetia gounellei White-bearded Hermit, Phaethornis hispidus Tawny-bellied Hermit, Phaethornis syrmatophorus Mexican Hermit, Phaethornis mexicanus Long-billed Hermit, Phaethornis longirostris Green Hermit, Phaethornis guy White-whiskered Hermit, Phaethornis yaruqui Great-billed Hermit, Phaethornis malaris Long-tailed Hermit, Phaethornis superciliosus Straight-billed Hermit, Phaethornis bourcieri Koepcke’s Hermit, Phaethornis koepckeae Needle-billed Hermit, Phaethornis philippii Buff-bellied Hermit, Phaethornis subochraceus Scale-throated Hermit, Phaethornis eurynome Sooty-capped Hermit, Phaethornis augusti Planalto Hermit, Phaethornis pretrei Pale-bellied Hermit, Phaethornis anthophilus Stripe-throated Hermit, Phaethornis striigularis Gray-chinned Hermit, Phaethornis griseogularis Black-throated Hermit, Phaethornis atrimentalis Reddish Hermit, Phaethornis ruber ?White-browed Hermit, Phaethornis stuarti ?Dusky-throated Hermit, Phaethornis squalidus Streak-throated Hermit, Phaethornis rupurumii Cinnamon-throated Hermit, Phaethornis nattereri Little Hermit, Phaethornis longuemareus ?Tapajos Hermit, Phaethornis aethopygus ?Minute Hermit, Phaethornis idaliae Polytminae: Mangos Lesbiini: Coquettes Lesbiinae Coeligenini: Brilliants Patagonini: Giant Hummingbird Lampornithini: Mountain-Gems Tro chilinae Mellisugini: Bees Cynanthini: Emeralds Trochilini: Amazilias Source: McGuire et al. (2014).. -

The Migration Strategy, Diet & Foraging Ecology of a Small
The Migration Strategy, Diet & Foraging Ecology of a Small Seabird in a Changing Environment Renata Jorge Medeiros Mirra September 2010 Thesis submitted for the degree of Doctor of Philosophy, Cardiff School of Biosciences, Cardiff University UMI Number: U516649 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. Dissertation Publishing UMI U516649 Published by ProQuest LLC 2013. Copyright in the Dissertation held by the Author. Microform Edition © ProQuest LLC. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 Declarations & Statements DECLARATION This work has not previously been accepted in substance for any degree and is not concurrently submitted in candidature for any degree. Signed j K>X).Vr>^. (candidate) Date: 30/09/2010 STATEMENT 1 This thasjs is being submitted in partial fulfillment of the requirements for the degree o f ..................... (insertMCh, MD, MPhil, PhD etc, as appropriate) Signed . .Ate .^(candidate) Date: 30/09/2010 STATEMENT 2 This thesis is the result of my own independent work/investigation, except where otherwise stated. Other sources are acknowledgedjjy explicit references. Signe .. (candidate) Date: 30/09/2010 STATEMENT 3 I hereby give consent for my thesis, if accepted, to be available for photocopying and for inter-library loan, and for the title and summary to be made available to outside organisations. -

The Cycle of the Common Loon (Brochure)
ADIRONDACK LOONS AND LAKES FOR MORE INFORMATION: NEED YOUR HELP! lthough the Adirondack Park provides A suitable habitat for breeding loons, the summering population in the Park still faces many challenges. YOU CAN HELP! WCS’ Adirondack Loon Conservation Program Keep Shorelines Natural: Help maintain ~The Cycle of the this critical habitat for nesting wildlife and 7 Brandy Brook Ave, Suite 204 for the quality of our lake water. Saranac Lake, NY 12983 Common Loon~ (518) 891-8872, [email protected] Out on a Lake? Keep your distance (~100 feet or more) from loons and other wildlife, www.wcs.org/adirondackloons so that you do not disturb them. The Wildlife Conservation Society’s Adirondack Going Fishing? Loon Conservation Program is dedicated to ∗ Use Non-Lead Fishing Sinkers and improving the overall health of the environment, Jigs. Lead fishing tackle is poisonous to particularly the protection of air and water loons and other wildlife when quality, through collaborative research and accidentally ingested. education efforts focusing on the natural history ∗ Pack Out Your Line. Invisible in the of the Common Loon (Gavia immer) and water, lost or cut fishing line can conservation issues affecting loon populations entangle loons and other wildlife, often and their aquatic habitats. with fatal results. THE WILDLIFE CONSERVATION SOCIETY IS Be an Environmentally Wise Consumer: GRATEFUL TO ITS COLLABORATORS FOR THEIR Many forms of environmental pollution SUPPORT OF THE LOON PROGRAM: result from the incineration of fossil Natural History Museum of the Adirondacks - fuels, primarily from coal-fired power The W!ld Center plants and vehicles, negatively affecting www.wildcenter.org A guide to the seasonal Adirondack ecosystems and their wild NYS Dept. -

The Effect of Intense Light on Bird Behavior and Physiology
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DigitalCommons@University of Nebraska University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Wildlife Damage Management, Internet Center Bird Control Seminars Proceedings for October 1973 THE EFFECT OF INTENSE LIGHT ON BIRD BEHAVIOR AND PHYSIOLOGY Sheldon Lustick Ohio State University Follow this and additional works at: https://digitalcommons.unl.edu/icwdmbirdcontrol Part of the Environmental Sciences Commons Lustick, Sheldon, "THE EFFECT OF INTENSE LIGHT ON BIRD BEHAVIOR AND PHYSIOLOGY" (1973). Bird Control Seminars Proceedings. 119. https://digitalcommons.unl.edu/icwdmbirdcontrol/119 This Article is brought to you for free and open access by the Wildlife Damage Management, Internet Center for at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Bird Control Seminars Proceedings by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 171 THE EFFECT OF INTENSE LIGHT ON BIRD BEHAVIOR AND PHYSIOLOGY Sheldon Lustick Zoology Department Ohio State University , Columbus , Ohio 43210 It has been known for centuries that light (photoperiod ) is possibly the major environmental stimuli affecting bird behavior and physiology. The length of the light period stimulates the breeding cycle , migration , fat de - position , and molt in most species of birds. Therefore , it is only natural that one would think of using light as a means of bird control. In fa ct , light has already been used as a bird control; flood -light traps have been used to trap blackbirds (Meanley 1971 ); Meanley states that 2000 -W search lights have been used to alleviate depredation by ducks in rice fields. -

GCE Biology Question Paper Unit 02
For Examiner’s Use Centre Number Candidate Number Surname Other Names Examiner’s Initials Candidate Signature Question Mark General Certificate of Education 1 Advanced Subsidiary Examination June 2012 2 3 Biology BIOL2 4 Unit 2 The variety of living organisms 5 6 Monday 21 May 2012 1.30 pm to 3.15 pm 7 8 For this paper you must have: l a ruler with millimetre measurements. 9 l a calculator. TOTAL Time allowed l 1 hour 45 minutes Instructions l Use black ink or black ball-point pen. l Fill in the boxes at the top of this page. l Answer all questions. l You must answer the questions in the spaces provided. Do not write outside the box around each page or on blank pages. l You may ask for extra paper. Extra paper must be secured to this booklet. l Do all rough work in this book. Cross through any work you do not want to be marked. Information l The maximum mark for this paper is 85. l You are expected to use a calculator, where appropriate. l The marks for questions are shown in brackets. l Quality of Written Communication will be assessed in all answers. l You will be marked on your ability to: – use good English – organise information clearly – use scientific terminology accurately. (JUN12BIOL201) WMP/Jun12/BIOL2 BIOL2 Do not write 2 outside the box Answer all questions in the spaces provided. 1 (a) Flatworms are small animals that live in water. They have no specialised gas exchange or circulatory systems. The drawing shows one type of flatworm. -

Brazil's Eastern Amazonia
The loud and impressive White Bellbird, one of the many highlights on the Brazil’s Eastern Amazonia 2017 tour (Eduardo Patrial) BRAZIL’S EASTERN AMAZONIA 8/16 – 26 AUGUST 2017 LEADER: EDUARDO PATRIAL This second edition of Brazil’s Eastern Amazonia was absolutely a phenomenal trip with over five hundred species recorded (514). Some adjustments happily facilitated the logistics (internal flights) a bit and we also could explore some areas around Belem this time, providing some extra good birds to our list. Our time at Amazonia National Park was good and we managed to get most of the important targets, despite the quite low bird activity noticed along the trails when we were there. Carajas National Forest on the other hand was very busy and produced an overwhelming cast of fine birds (and a Giant Armadillo!). Caxias in the end came again as good as it gets, and this time with the novelty of visiting a new site, Campo Maior, a place that reminds the lowlands from Pantanal. On this amazing tour we had the chance to enjoy the special avifauna from two important interfluvium in the Brazilian Amazon, the Madeira – Tapajos and Xingu – Tocantins; and also the specialties from a poorly covered corner in the Northeast region at Maranhão and Piauí states. Check out below the highlights from this successful adventure: Horned Screamer, Masked Duck, Chestnut- headed and Buff-browed Chachalacas, White-crested Guan, Bare-faced Curassow, King Vulture, Black-and- white and Ornate Hawk-Eagles, White and White-browed Hawks, Rufous-sided and Russet-crowned Crakes, Dark-winged Trumpeter (ssp. -

How to Participate in the Fairywren Project
How to participate in the Fairywren Project Step 1: Log in to eBird Step 2: Choose a participation level and contribute The Fairywren Project is interested in many aspects of fairywren behavior and ecology. We’ve broken down our data-collection into three levels of participation, beginning with the most important way to contribute. Whether you choose to participate at the basic, intermediate, or advanced level, any data you submit to eBird is a significant contribution! Basic Participation: Which plumage types are you seeing? The simplest, but by far the most important way to contribute to the project is to let us know what species and plumage types you’re seeing. We’ve divided plumage into five categories, one for females, three for males, and one for dull birds of unknown sex: Bright Male Plumage (b): A bright bird is one that is in full flashy breeding plumage and does not obviously show any dull feathers intermixed with bright feathers. A species bright plumage is typically the plumage the species is known and named for. Only males exhibit bright plumage. Intermediate Male Plumage (i): Intermediate plumage is a male in any stage between dull (no bright feathers) and completely bright (all bright feathers). An intermediate male may be actively molting, but some species are known to maintain intermediate plumages for months at a time. Dull Male Plumage (d): Dull male plumage is the species typical winter or non-breeding plumage (sometimes referred to as “eclipse” plumage). A male in dull plumage should show no bright body feathers, although some species like Superb Fairywrens often maintain blue tails year-round, even when in dull plumage. -

Magnificent Magpie Colours by Feathers with Layers of Hollow Melanosomes Doekele G
© 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb174656. doi:10.1242/jeb.174656 RESEARCH ARTICLE Magnificent magpie colours by feathers with layers of hollow melanosomes Doekele G. Stavenga1,*, Hein L. Leertouwer1 and Bodo D. Wilts2 ABSTRACT absorption coefficient throughout the visible wavelength range, The blue secondary and purple-to-green tail feathers of magpies are resulting in a higher refractive index (RI) than that of the structurally coloured owing to stacks of hollow, air-containing surrounding keratin. By arranging melanosomes in the feather melanosomes embedded in the keratin matrix of the barbules. barbules in more or less regular patterns with nanosized dimensions, We investigated the spectral and spatial reflection characteristics of vivid iridescent colours are created due to constructive interference the feathers by applying (micro)spectrophotometry and imaging in a restricted wavelength range (Durrer, 1977; Prum, 2006). scatterometry. To interpret the spectral data, we performed optical The melanosomes come in many different shapes and forms, and modelling, applying the finite-difference time domain (FDTD) method their spatial arrangement is similarly diverse (Prum, 2006). This has as well as an effective media approach, treating the melanosome been shown in impressive detail by Durrer (1977), who performed stacks as multi-layers with effective refractive indices dependent on extensive transmission electron microscopy of the feather barbules the component media. The differently coloured magpie feathers are of numerous bird species. He interpreted the observed structural realised by adjusting the melanosome size, with the diameter of the colours to be created by regularly ordered melanosome stacks acting melanosomes as well as their hollowness being the most sensitive as optical multi-layers. -

Grand Australia
GRAND AUSTRALIA PART II: QUEENSLAND, VICTORIA & PLAINS-WANDERER OCTOBER 12-29, 2020 TASMANIA EXTENSION OCTOBER 29-NOVEMBER 4, 2020* ©2020 *DATES DIFFER FROM THOSE THAT APPEAR IN OUR 2020 CATALOG This is the second part of our Grand Australia tour; VENT’s finest, most comprehensive tour of Australia. We will travel to several of the most important bird areas on the continent, searching out birds, mammals and wildflowers from the common to the rare and most elusive. We expect the field list from either of these parts to represent close to 50% of the species of Australia, which is half of Australia’s regular birds in two weeks of birding. We have designed all sections to allow flexibility for those who may have already enjoyed a birding trip to Australia, but missed some of the key sites. It also caters to those who have never visited Australia and wish to experience the whole spectrum of this diverse country by taking all three sections. Travelling in Australia is a delight; friendly people, excellent roads and hotels, reliable airlines, plenty of fresh produce and tasty food. You can drink the water and eat the salads! On this tour we make a number of two and even three-night stays and travel in a vehicle that affords excellent viewing and an abundance of seats! Australia’s geographic isolation has resulted in the evolution of a remarkable array of endemic birds and other wildlife. More than 300 species and eight families are endemic (making it one of the richest in the world). Colorful parrots, fairywrens, finches and honeyeaters light up the environment while giant Cassowary and Emu, peculiar bowerbirds and birds-of-paradise make their home down under. -

New Guinea & Australia 2016
Field Guides Tour Report New Guinea & Australia 2016 Oct 27, 2016 to Nov 14, 2016 Jay VanderGaast & Doug Gochfeld For our tour description, itinerary, past triplists, dates, fees, and more, please VISIT OUR TOUR PAGE. The amazing Wattled Ploughbill, a Papuan endemic, photographed by guide Doug Gochfeld. As the snow falls outside and winter envelops my home, it's fun to look back on this wonderful tour and recall the tropical heat and all the hot birding that took place during our time in PNG and Australia. It was a whirlwind couple of weeks in which we visited some of eastern Australia's premier birding hotspots, sandwiched around a brief visit to a couple of choice destinations in PNG. The results were satisfying, as we enjoyed a great selection of the region's unique birds, including representatives of a number of families restricted to this part of the world, from Emus and brushturkeys to bowerbirds, fairywrens, and of course, birds-of-paradise. We began our adventure in the steamy tropics of Far North Queensland, arriving in the friendly coastal city of Cairns, which we used as a base to explore the surrounding, bird-rich areas. Our first afternoon of birding just to the north of the city and along the famous Esplanade gave us our first taste of the treats in store for us, with Green Pygmy-Goose, Magpie Goose, Orange-footed Scrubfowl, Rainbow Bee-eaters, and Crimson Finches starting things off right at Yorkey's Knob and Cattana Wetlands, and the high tide along the Esplanade pushing a plethora of shorebirds within easy viewing distance. -

Common Birds of the Estero Bay Area
Common Birds of the Estero Bay Area Jeremy Beaulieu Lisa Andreano Michael Walgren Introduction The following is a guide to the common birds of the Estero Bay Area. Brief descriptions are provided as well as active months and status listings. Photos are primarily courtesy of Greg Smith. Species are arranged by family according to the Sibley Guide to Birds (2000). Gaviidae Red-throated Loon Gavia stellata Occurrence: Common Active Months: November-April Federal Status: None State/Audubon Status: None Description: A small loon seldom seen far from salt water. In the non-breeding season they have a grey face and red throat. They have a long slender dark bill and white speckling on their dark back. Information: These birds are winter residents to the Central Coast. Wintering Red- throated Loons can gather in large numbers in Morro Bay if food is abundant. They are common on salt water of all depths but frequently forage in shallow bays and estuaries rather than far out at sea. Because their legs are located so far back, loons have difficulty walking on land and are rarely found far from water. Most loons must paddle furiously across the surface of the water before becoming airborne, but these small loons can practically spring directly into the air from land, a useful ability on its artic tundra breeding grounds. Pacific Loon Gavia pacifica Occurrence: Common Active Months: November-April Federal Status: None State/Audubon Status: None Description: The Pacific Loon has a shorter neck than the Red-throated Loon. The bill is very straight and the head is very smoothly rounded. -

Brachylaimid and Dicrocoeliid Trematodes of Birds from North Borneo (Malaysia)1
94 PROCEEDINGS OF THE HELMINTHOLOGICAL SOCIETY Brachylaimid and Dicrocoeliid Trematodes of Birds from North Borneo (Malaysia)1 JACOB H. FISCHTHAL AND ROBERT E. KuNTZ2 ABSTRACT: One brachylaimid and 10 dicrocoeliid trematodes of birds are reported from North Borneo (Malaysia). New species described are Brachylaima (Brachylaima) sabahense, Brachydistomiim api, Brachylecithum pycnonoti, B. sabahense, B. vitellobum, and Lyperosomum malaysiae. Briefly described are Brachylecithum attenuatum (Dujardin, 1845) Shtrom, 1940, Lutztrema bhattacharyai (Pande, 1939) Travassos, 1944, and Proacetabidorchis dogieli Belopolskaja and Bykhovskaja-Pavlovskaja, 1953. Also reported are Athesmia heterolecithodes (Braun, 1899) Looss, 1899, and Proacetabiilorchis prashadi Gogate, 1940. The trematodes of this paper are part of a Family Brachylaimidae collection made by the junior author while a Brachylaima (Brachylaima) member of the U. S. Naval Medical Research sabahense sp. n. Unit No. 2, Taipei, Taiwan. Parasites were washed in saline, killed in hot water, and trans- (Figs. 1, 2) ferred immediately to FA A fixative; after 4 HOSTS: Type, Aplonis panayensis (Scopali), to 8 hr they were stored in 70% alcohol plus starling (Passeriformes: Sturnidae); Orthoto- 2% glycerin. Staining was in Mayer's carm- miifi sepium bomeoensis Salvador!, red-headed tailor bird (Passeriformes: Muscicapiclae: Sylvi- alum, and all were mounted in Permount. Host nae); Nyctyornis amictus (Temminck), red- names recorded herein are those listed by bearded bee-eater (Coraciiformes: Meropidae). Kuntz (1969). Host names preceded by an HABITAT: Small intestine. asterisk ('::") represent new host records. Speci- LOCALITIES: Kasiqui, Petergas. mens of each trematode species reported have DATES: 3, 16 September 1960. been deposited in the U. S. National Museum SPECIMENS DEPOSITED: No. 72713 (holo- Helminthological Collection as noted.